| Pages:
1
..
14
15
16
17
18
..
33 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Darkstar  | | a brief introduction to moieties and functional groups. I drew some random molecules with a bunch of functional groups on them and wrote their names
next to them. I also threw in a little refresher on skeletal structures as well. I hope you're not color blind... |
Nope. Not colour blind.
What a WONDERFUL explanation of what all those magic words mean !
Thank you very much indeed Darkstar
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Answers to Questions 3 and 4:
A.3:
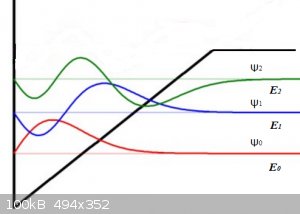
All three wave functions are zero at x < or = 0 because there V = +∞.
For the ground state ψ<sub>0</sub> the wave function (red) takes on a sine (or bell) shaped form with no zero-points, until the potential
V > E<sub>0</sub>, then the wave function tends to 0 for x tending to +∞.
For the first excitation ψ<sub>1</sub> the wave function (blue) must exhibit one zero point as shown, then when the potential V >
E<sub>1</sub>, then the wave function tends to 0 for x tending to +∞.
For the second excitation ψ<sub>2</sub> the wave function (green) must exhibit two zero points as shown, then when the potential V >
E<sub>2</sub>, then the wave function tends to 0 for x tending to +∞.
This particular potential shape is a crude approximation of the often encountered Morse Potential.
A.4:
There is only one naphtalene and one 2,3 dichloro naphthalene due to resonance.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
| Quote: | | with no zero-points, until the potential V > E0, then the wave function tends to 0 for x tending to +∞ |
Confused.
The E0 waveform never gets anywhere near V=0, unless '0' means zero deviation from the ground state, in which case it starts there, so has
at least 2 (start and where it goes flat).
Are the bits past the right hand box wall tunnelling ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Erratum:
Oooopsie, a typo in Question 6.
It should be about dinitrogen difluoride, N<sub>2</sub>F<sub>2</sub>, of course.
Apologies.
[Edited on 2-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Armed with Darkstar's Guide to OC Magik i've picked these three chem names to decipher (from the today's posts list)
#1 - 1,3 propanediol
#2 - Benzotriazole
#3 - Triethylamine
Had to look up what 'iol' meant, and found that 'diol' means two Hydroxyl (OH) groups. The 1,3 are the carbon positions, and it's so short there can't
really be much question as to which number goes where.
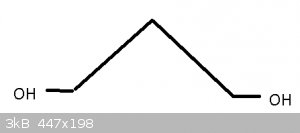
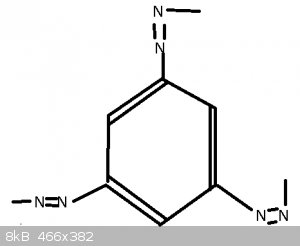
With Benzotriazole, not sure if this is right, seeing as it's not 'triazo' so perhaps the 'le' means something more.
Also unclear why it's not called 'Phenotriazo' seeing as the ring looks the same.
Triethylamine was trickier.
Tried a string of ether segments in a chain with the amine on the end before looking up Nitrogen on ptable.com to find it wants 3 bonds, so the 'tri'
kinda means it chucks the H for C, although that makes it not 'amine' as per the clue sheet.
The 'ethyl' instead of 'ether' also makes me suspect that this one is very wrong.
Edit:
Oops. Put Nitriles on the Benzo instead of Azos.
[Edited on 2-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | | Quote: | | with no zero-points, until the potential V > E0, then the wave function tends to 0 for x tending to +∞ |
Confused.
The E0 waveform never gets anywhere near V=0, unless '0' means zero deviation from the ground state, in which case it starts there, so has
at least 2 (start and where it goes flat).
Are the bits past the right hand box wall tunnelling ?
|
Look at the reddish horizontal line (E<sub>0</sub> , at some point it
(call it x<sub>0</sub> , at some point it
(call it x<sub>0</sub> crosses the V(x) line. From then on (for x >
x<sub>0</sub> crosses the V(x) line. From then on (for x >
x<sub>0</sub> V(x) > E<sub>0</sub>. In that region we
get tunnelling. The wave function decays exponentially to ψ = 0 for larger and larger x. V(x) > E<sub>0</sub>. In that region we
get tunnelling. The wave function decays exponentially to ψ = 0 for larger and larger x.
The same holds for the blue and green cases but their 'crossing points' lie at higher values of x.
[Edited on 3-11-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Armed with Darkstar's Guide to OC Magik i've picked these three chem names to decipher (from the today's posts list)
#1 - 1,3 propanediol
#2 - Benzotriazole
#3 - Triethylamine
|
Slightly smoother version of 1,3 propanediol and triethylamine (no oxygens):
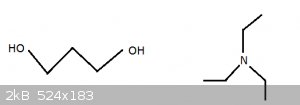
[Edited on 2-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Further confused !
Where did the oxygens go ?
Edit:
Triethylamine should be made on the Isle of Man 
[Edited on 2-11-2015 by aga]

|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
They must have stayed on the Isle of Man:
https://en.wikipedia.org/wiki/Triethylamine
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Here are the molecules in question:
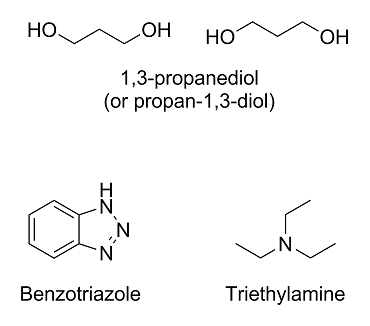
The first one, 1,3-propanediol (under IUPAC nomenclature rules, it would technically be propan-1,3-diol), you got right; however, it will usually be
drawn like above.
The second one, benzotriazole, is a lot harder since we have covered neither nomenclature rules nor heterocyclic compounds. The molecule is basically
benzene fused with 1,2,3-triazole.
The last one, triethylamine, is a nitrogen with three ethyl (not ether!) groups on it. The ethyl groups are just ethane molecules with one less
hydrogen on them. The eth- prefix means that it's a chain with two carbons, and the -yl suffix means that a hydrogen has been replaced with something
else.
Unfortunately, I'm a little pressed for time at the moment, so I can't elaborate further. I'll probably do a section later today covering some basic
nomenclature rules and show you some of the common heterocylics. It's awesome that you're at least trying to apply what you're learning, though. Your
teachers are very proud!
EDIT - Fixed a mistake I made in 1,3-propanediol's IUPAC name. Was in a hurry when making the image and just copied and pasted the "1,3-propanediol"
while I was adding the text below the molecules in ChemDraw. It is all fixed now.
[Edited on 11-2-2015 by Darkstar]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by aga  | | Tried a string of ether segments in a chain with the amine on the end before looking up Nitrogen on ptable.com to find it wants 3 bonds, so the 'tri'
kinda means it chucks the H for C, although that makes it not 'amine' as per the clue sheet. |
Just a quick note, there are basically three kinds of non-cyclic amines: primary, secondary and tertiary.
Primary = One hydrogen replaced
Secondary = Two hydrogens replaced
Tertiary = Three hydrogens replaced
Thus triethylamine is said to be a tertiary amine. And while not technically amines (but often formed from them), there are also positively-charged
quaternary ammonium ions, which are ions where a nitrogen has all three hydrogens replaced as well as a bond using its lone pair, making a total of
four bonds. (like in ammonium nitrate)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It might also help aga to understand that skeletal notations do have another bearing in reality.
Have a look at the diagram below. Left are the ball-and-stick representations of the first 3 alkanes and right their skeletal representations:
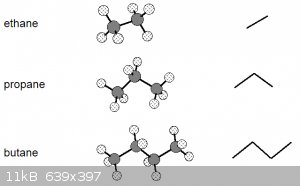
Note that the bond angles in the ball and stick models are to scale (true angles) and that the skeletal representation is essentially the projection
of the C nuclei (the hydrogens aren't shown unless necessary) and the lines representing the bonding orbitals onto a horizontal plane, see
below for butane:
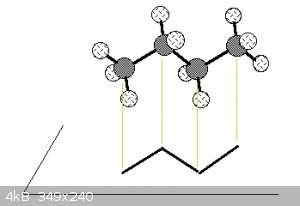
Which will bring me to a later subject: molecular conformations.
[Edited on 3-11-2015 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Excellent post, blogfast. I probably should have mentioned that skeletal structures are drawn the way they are for a reason. The "zigzagging" of the
lines isn't done simply because it looks good. I guess I should have made that part "explicit."
Speaking of stereoisomerism, I was actually thinking about doing a brief introduction on chirality soon. So maybe you do conformers and I'll do
enantiomers? And then whoever's up for it can do one on cis-trans isomerism and finally one on how to name all of these different types of
stereoisomers using R/S and E/Z notation.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  |
Speaking of stereoisomerism, I was actually thinking about doing a brief introduction on chirality soon. So maybe you do conformers and I'll do
enantiomers? And then whoever's up for it can do one on cis-trans isomerism and finally one on how to name all of these different types of
stereoisomers using R/S and E/Z notation. |
It's a deal (especially since as I get the easier part, haha). But I must say my post on conformers will be really short, with a few interesting
examples like cyclohexane and maybe a couple of bicyclos.
One thing that cannot be excluded in a 'webinar' about QChemistry is OC IR spectroscopy. Again, something short and succinct would be appropriate.
Thanks again for all your help here.
[Edited on 3-11-2015 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I know this thread is supposed to be geared more towards QM's application to chemistry and not pure OC, so I don't mean to turn this into the official
"Teach Aga Organic Chemistry" thread. (though I did make a few QM-related contributions as well). It's just that I don't know where else to post this
stuff without making an entirely separate thread for it. And since what you're teaching him is often readily applicable to what I'm teaching him, and
the fact that he's extremely active in this thread and checking it is probably one of the very first things he does when he comes online, I see no
better place to do it. Not to mention that you're also here and can assist in answering his questions. Which is also why I like this whole
"interactive seminar" idea. Even though this information is all over the internet and can be found with a quick Google search, the fact that people
can actively ask questions when they don't quite understand something is nice. I suppose I could always just do it via U2U, but then that would
prevent anyone else from being able to benefit from it in the future.
Aga often talks about how impossible it is to learn OC and I want to finally show him once and for all that it's not. And as long as he seems
genuinely interested and eager to learn, I'm more than happy to help teach him. Even if he's the only person that ends up benefiting from any of this,
I'm perfectly okay with continuing to contribute. I was very pleased to see him try to draw those molecules earlier, especially since he got one of
them right without any real introduction to nomenclature. Besides, if nothing else, these lessons give me an excuse to play around in ChemDraw.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
This thread certainly is one of the first things i check when i go online !
The QM/OC mix is not confusing - it is in fact really beneficial.
I've never learnt so much from 1 thread before !
(likely many others are lurking and learning too > 8900 reads)
Thank you both very much for your time and patience.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Molecular conformations:
Isomers:
Two chemical compounds are said to be isomers is they have the same molecular formula but a different structure. Consider the simple case of n-butane
(“n” for “normal”, left) and isobutane (IUPAC: methyl propane, right):

Both have the empirical formula C<sub>3</sub>H<sub>8</sub>, yet structurally they are different. Both compounds have indeed
slightly different physical and chemical properties.
Now with general alkanes – C<sub>n</sub>H<sub>2n+2</sub> – we can have a lot of fun.
Take a look at some isomers of heptane C<sub>7</sub>H<sub>16</sub>:
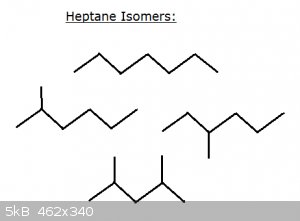
Top: n-heptane
Middle: 2-methyl hexane and 3-methyl hexane
Bottom: 2,4-dimethyl pentane
Now imagine writing all the isomers of decane – C<sub>10</sub>H<sub>22</sub> – or higher C. Thanks to carbon’s
unparalleled ability to form covalent bonds with itself and hydrogen (and other elements), isomers are very common in organic chemistry.
Molecular Conformations:
Let’s go back to these ball-and-stick models of the first three alkanes:
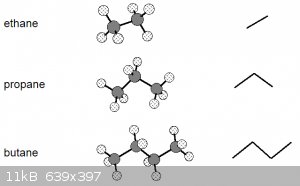
These models suggest a certain rigidity that isn’t really there. Due to the nature of covalent single σ bonds, the carbon nuclei
(and the atoms bonded to them) can be rotated around the inter-nuclear axis, leading to a number of different shapes, known as conformations.
The diagram above thus really shows the molecules in only one of their conformations.
The principle of conformations is very nicely explained (in under two minutes!) in the video below:
https://www.youtube.com/watch?v=yBtMjTZrMFc
It might be tempting to see molecular conformations of the same molecule also as isomers but that’s not really true.
A particularly interesting case is cyclohexane, in skeletal notation depicted as a regular hexagon. But due to bond angles that’s far from a
realistic representation:
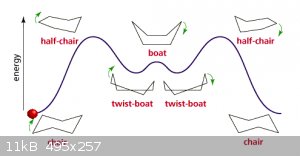
The source page contains a java script that doesn’t work on my tooter but it might on yours.
The energy levels are caused by various ‘stresses’ introduced into the molecule as it transforms from ‘left chair’ to ‘right chair’. As
the chair conformation is the lowest energy level liquid cyclohexane can be expected to be made up of mostly that conformation.
Finally, and to get Darkstar on his way to expand on chiral isomers, an interesting bicyclo compound, named alpha pinene:
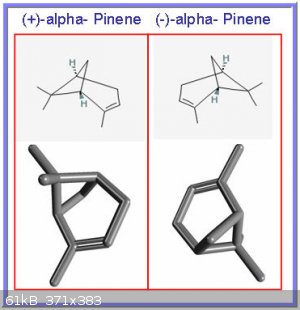
A ‘distant cousin’ of cyclohexane, alpha pinene has a carbon bridge connection that creates a secondary cyclic structure (hence “bicyclo”). It
has two permanent and distinct main conformations (termed (+) and(-)) that are mirror images of each other. The two forms are so-called
enantiomers.
[Edited on 3-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Hmm.
Watching the video, when he twisted the left hand part (as seen on screen), that brought the hydrogens on the left-hand end black ball into much
closer proximity to those attached to the green ball on the right.
Presumably all molecules take on a stable form based on where the electronegativity and bonding orbitals of all the atoms are best balanced ?
Assuming further, given the sheer size of some of these OC molecules, the distance between some atoms (e.g. at either end of a long chain) would make
those charges irrelevant - they;d never be able to get close enough to make a difference.
| Quote: | | I was very pleased to see him try to draw those molecules earlier |
It was fun to be able to even attempt them, armed with the new insights you've given !
Side note to those scared of getting things wrong and looking as un-cool-as-aga :-
Naturally i googled them after, and could have copy-n-pasted the correct structures, although that would not reveal my defective
understanding, and it'd not help me learn anything at all.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Hmm.
Watching the video, when he twisted the left hand part (as seen on screen), that brought the hydrogens on the left-hand end black ball into much
closer proximity to those attached to the green ball on the right.
Presumably all molecules take on a stable form based on where the electronegativity and bonding orbitals of all the atoms are best balanced ?
Assuming further, given the sheer size of some of these OC molecules, the distance between some atoms (e.g. at either end of a long chain) would make
those charges irrelevant - they;d never be able to get close enough to make a difference.
|
Good reasoning.
"where the electronegativity and bonding orbitals of all the atoms are best balanced" is a little woolly. "Where mutual electrostatic
repulsion between bonding orbitals is minimised" is a better way of putting it.
Where there's little difference in energy between conformations, all will occur in the real compound (the gas or liquid) because of constant
collisions between the molecules.
You're correct that in larger structures 'one end' is largely uninfluenced by the 'other end'.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks for the shearing.
Will try to remember the 'mutual electrostatic repulsion between bonding (and presumably non-bonding) orbitals' thing.
Is it just me, or is this thread the most focussed, direct and clear explanation of Key Chemistry Knowledge there has ever been ?
Certainly works for me : long may it continue !
Edit:
The odd thing is that the thread reads like there's just a few participants, yet it feels like there's a very large audience avidly waiting to see
what will happen.
[Edited on 3-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Here at Blog & Darkstar Educational Services we live by our motto: 'Educate first, Bill later'.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sounds fair.
Send me the bill when i'm demonstrably as knowledgeble as both of you 
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
@aga
Sometime later today when I have a little more time I'll post your next lesson. For now, it is time for your very first OC pop quiz. I have prepared
the following for you:
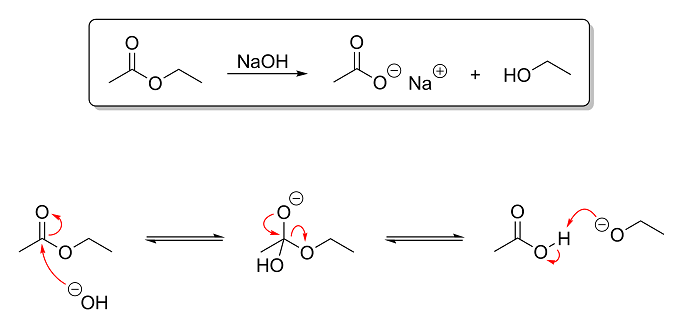
The reaction above is an example of base-catalyzed ester hydrolysis. In this reaction, ethyl acetate is hydrolyzed using aqueous NaOH to produce
sodium acetate (a salt) and ethanol. This reaction is the opposite of esterification (which we looked at earlier) and is the basis of soap-making. Triglycerides (fats) are essentially glycerin with ester groups instead of hydroxyl groups. Since I remember you saying that you have done saponification before, I figured this
reaction would be a good one to show you next. So based on what you've learned thus far, here's what I want you to tell me:
Question 1 - Identify the electrophile and nulcleophile in step one of the mechanism and the electrophile and nulcleophile in step
three of the mechanism. It is absolutely critical that you grasp this concept. Being able to properly differentiate between electrophiles and
nucleophiles is quite possibly the single most important aspect of understanding OC reactions!
Hint: electrophiles are electron-deficient and consequently attract nucleophiles, which are electron-hungry.
Question 2 - Briefly explain what is going on in each step of the mechanism. The level of depth I went into in my post on the Fischer
esterification is not necessary. One or two sentences per step describing what is reacting with what (and preferably why and how) is all that I am
looking for.
Extra Credit - For extra credit, tell me why this reaction is more or less irreversible under basic conditions. Notice that, while I
used equilibrium arrows in the mechanism steps to show that they are reversible, in the overall reaction at the top, a normal arrow is used instead.
This is because, after the third and last step of the mechanism, the reaction can no longer be undone without first lowering the pH. So in other
words, tell me why the reaction is no longer reversible after the final step of the mechanism.
Hint: why is it reversible before the ethoxide ion is protonated but not after? An ethoxide ion is ethanol without a proton
on its hydroxyl group, by the way.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sheesh ! i almost forgot there was another question and it's mid-week already ! 
Edit:
WTF ?!?!
As soon as i finished typing that there's two new Darkstar Questions !
Right.
Game On.
[Edited on 4-11-2015 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Are the OC answers open to everyone, and should be U2U'd to you Darkstar, same as for the QM answers to blogfast ?
|
|
|
| Pages:
1
..
14
15
16
17
18
..
33 |