| Pages:
1
..
24
25
26
27
28
..
33 |
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
That's an interesting idea, I've never heard of a reaction being affected by ring currents. Using a Helmholtz coil would produce a significantly more
constant and stable magnetic field, rather than a fixed magnet. I can't think of any reactions right which would necessarily be worth a try, perhaps
blogfast has an idea.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Being a mere novice, i have no idea.
Is there an OC reaction (involving resonance/ring structures) that takes a particular pathway/mechanism every single time, despite the possibility of
other pathways ?
If so, then some experiments may be worthwhile.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
I have Sodium Benzoate and some Huge Nd magnets.
Suggestions for a Practical reaction that generally goes One route, and might go another under such circumstances ?
Perhaps a large magnetic field plus electrolysis. |
Erm... have you seen the magnets on these NMR gismoz:
https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spe...

|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
This last page has me wondering, does this mean those gas magnet inventions may actually have some basis in theory for the claimed ability to increase
combustion efficiency (ring currents).
[Edited on 12-5-2015 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
With/without magnetic field experiments on OC with ring structures is kinda the idea ...
Do not be too hard on Pok.
He's not posted any new topics recently, so it may just be jealousy.
[Edited on 4-12-2015 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Inefficiency is not impressive.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by IrC  | This last page has me wondering two things. One is does this mean those gas magnet inventions may actually have some basis in theory for the claimed
ability to increase combustion efficiency (ring currents).
|
I don't really get your question. 'Splain?
The magnetic fields needed to do NMR are far, far higher than your neomags can deliver.
[Edited on 4-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Of course.
Now consider why that would be the case.
Why would an Enormous magnetic field be required to affect the passage of a single atom, when the self-evident fact is that a much much weaker
magnetic field can stick a magnet to Iron so hard that a human lacks the strength to remove it ?
QM was invented to solve Other problems.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
"I don't really get your question. 'Splain?"
I have read many articles on those devices sold to apply a strong local magnetic field to gas going into an engine, both pro and con. Some of the pro
articles did quite a bit of testing MPG over the same track both with and without the device installed. Since it sounds as if what is being said was
molecules with ring currents can be affected by magnetic fields, it makes me wonder if similar to a catalyst altering the activation energy level, or
some type of molecular rearrangement may be induced by the field which improves the combustion process. Up to this point I had always considered the
magnet on the fuel line to be more or less snake oil. This ring current discussion where magnetic fields may cause some change sparked my memory of
looking into those devices decades ago. Is it possible the oil may not all be snakes or could some process little understood and yet to be researched
fully actually be involved? In other words should I buy snake traps or N52 magnets?
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Well, according to the SE the magnetic dipole moment for a hydrogen atom in the eigenstate (n,l,m) is quantised and given by the following equations:
$$\mu_L=-g_L\frac{\mu_B}{\hbar}\langle \Psi_{n,l,m}|L|\Psi_{n,l,m} \rangle=-\mu_B\sqrt{l(l+1)}.$$
$$(\mu_L)_z=-\mu_B m.$$
$$n=0,1,2,3,...$$
$$l=0,1,..., n-1$$
$$m=-l,-l+1,...,0,...,+l-1,+l$$
$$\mu_B=\frac{e\hbar}{2m_e}=9.274 \times 10^{-24}\:\mathrm{JT^{-1}}$$
(μ<sub>B</sub> <sub>z</sub> is the z component and
μ<sub>B</sub> the Bohr magneton. <sub>z</sub> is the z component and
μ<sub>B</sub> the Bohr magneton.
To this then has to be vector-added the intrinsic spin magnetic dipole moment of an electron (spin 'up', spin 'down', remember?).
So we're talking pretty small numbers here.
[Edited on 5-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by IrC  | "I don't really get your question. 'Splain?"
I have read many articles on those devices sold to apply a strong local magnetic field to gas going into an engine, both pro and con. Some of the pro
articles did quite a bit of testing MPG over the same track both with and without the device installed. Since it sounds as if what is being said was
molecules with ring currents can be affected by magnetic fields, it makes me wonder if similar to a catalyst altering the activation energy level, or
some type of molecular rearrangement may be induced by the field which improves the combustion process. Up to this point I had always considered the
magnet on the fuel line to be more or less snake oil. This ring current discussion where magnetic fields may cause some change sparked my memory of
looking into those devices decades ago. Is it possible the oil may not all be snakes or could some process little understood and yet to be researched
fully actually be involved? In other words should I buy snake traps or N52 magnets?
|
Oh yeah, I remember a bit of brouhaha around these. I'm not an expert by a long shot on these issues IrC, but my money is on smelling snake
oil. And I never buy into 'it wos Big Oil wot killed it' type of arguments.
I've never really heard of magnetic fields significantly affecting reaction outcomes at all (but there's a first time for everything, maybe?)
[Edited on 5-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
And here's a suggestion for an 'aga research project' in OC...
Firstly, you could just do some off-the-shelf synths: that's safe but a bit boring.
Or you (we) could do something a bit more off the beaten track with potential usefulness.
Here's the proposal.
Preparation of some saturated tertiary alcohols from α-pinene:
α-pinene is a fairly OTC material (natural turpentine contains quite a lot of it) with a skeletal structure that is found in many natural derivatives
(the result of evolutionary biology, no doubt).
Below are two α-pinene derivatives (4 and 6) that would be very well worth testing as catalysts in the KOH/Mg reduction reaction
(see pok's sticky thread).
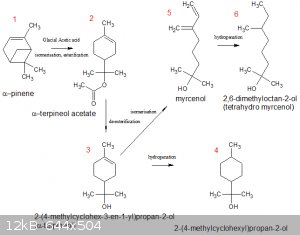
Preparing them from α-pinene seems doable but definitely not run-of-the-mill and with no guarantee of success.
The project would involve:
* Literature research (precursor and intermediates properties, recipes etc)
* Reactions
* Separations
* Characterisation of intermediates and end-products
* Reaction mechanism hypotheses
Needless to say: it'd keep you/us busy for a while!
aga, don't feel bamboozled into anything and make a free choice.
If you're interested, rest assured there will be plenty of assistance from the community.
Any questions?
PS: ultimate blackmail argument: 'you'd be giving something back to the community!'  
[Edited on 5-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cor blimey guv'ner !
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
An under-whelming response?
1 to 2 (one step), 2 to 3 (one step) and 3 to 4 (one step) are relatively straightforward. 3 to 5 is a bit of an unknown.
Anyroads, think it over. Perhaps other members may have an opinion too...
[Edited on 5-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
You reckon i can get anywhere near doing that chain of reactions with a > 0% probability of achieving any product at all ?
Over-awed and disbelieving is the response !
Turpentine, hmm, seeing as it's already bonkers, how about starting with pine resin ?
(lots of pine trees up in the mountains).
Edit:
Turning a Pine smell into a Lemon smell would be fun.
[Edited on 5-12-2015 by aga]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
The only issue I see with the suggested chain is the hydrogenation. They're a pain to do in an amateur setting as many of them need pressurized
hydrogen; at the very least some pricey transition metal catalysts are necessary (Pd/C). It would be a good endeavor to gain practical experience
though.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
How much hydrogen and how high a pressure ?
Blogger's reaction sequence obviously <i>has</i> to be done, now it's been suggested.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
If using Pd/C or Pt/C as a catalyst, you should be able to run the hydrogenation at atmospheric pressure, but you will need to generate dry hydrogen.
Borohydride and acid is the standard in professional labs, but I find it a waste of borohydride. The reaction of a metal such as iron with an acid
will generate hydrogen at a reasonably consistent rate; if the hydrogen is passed through a drying tube it should be of sufficient quality for this
reaction. The easiest way to transfer the hydrogen is via a balloon, so no trouble there. Once you get to that phase of the reaction, we can work
out a more precise procedure based on what equipment you have. If you decide to use Raney nickel as the catalyst, which can be prepared using
relatively simple aqueous chemistry, you will need a hydrogen cylinder and equipment which you trust to be under at least several atm of hydrogen
pressure, not a great situation if the glass fails.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
@bloggers: is the 2-(4-methylcyclohexyl)propan-2-ol (item #4) a <i>desired</i> product in this sequence, or is it a possible by-product
that should be minimised ?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by gdflp  | | will need a hydrogen cylinder and equipment which you trust to be under at least several atm of hydrogen pressure, not a great situation if the glass
fails. |
Does it have to be Glass ?
For high pressure/temperature i'd rather do some standard plumbing with steel.
Would the iron or other components of steel interfere ?
Edit:
"Pd/C or Pt/C" i do not know what that means.
Palladium or Platinum and Carbon is some form is the only sense i can make from the terms.
[Edited on 5-12-2015 by aga]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
And come to think of it, I don't know why I specifically said glass, an iron or steel pipe will work. It still seems like a pipe bomb to me though,
I'd rather use hydrogen at atmospheric pressure if at all possible. You can look up "Parr Hydrogenators" to see what a laboratory grade apparatus
designed for this looks like.
Yes Pd/C and Pt/C are shorthand for activated carbon impregnated with palladium or platinum metal. Typically, they are either 5% or 10% by weight
precious metal, with 5% being the most commonly used.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Activated Carbon !
I think i've heard of that somewhere ....
Given the number of variables i'd probably be better off just buying some, however this is Amateur Chemistry, so maybe better to make everything where
possible.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
If you decide to prepare it, OrgSyn has a detailed preparation here.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Superb !
A clear sign that What you Know can definitely be augmented by Who you know.
The choice of Route should really be up to the teaching staff, seeing as i'm just the spanner monkey, and you guys obviously know better than i.
If it means buying Platinum etc, no problem, just takes time to arrive is all.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
gdflp:
Lab hydrogenations can be carried out without actual hydrogen with Pd/C catalysts and ammonium formate as hydrogenation agent, see here:
http://oxfordchemserve.com/palladium-on-carbon-catalyst-pdc-...
(see third pic, here with formic acid)
I wouldn't have suggested this chain of synths w/o bearing that in mind. Pd/C catalysts aren't that expensive because they don't contain much Pd
anyway.
[Edited on 5-12-2015 by blogfast25]
|
|
|
| Pages:
1
..
24
25
26
27
28
..
33 |