| Pages:
1
..
25
26
27
28
29
..
33 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | | @bloggers: is the 2-(4-methylcyclohexyl)propan-2-ol (item #4) a <i>desired</i> product in this sequence, or is it a possible by-product
that should be minimised ? |
No 4 is an end product, as would be No 6. The desired t-alcohols are preferably fully saturated (no double bonds) because of the harsh conditions of
the KOH/Mg reduction.
But even just No3 as a clean product would be a worthy goal, should hydrogenation fail/prove too difficult.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Indeed, I was aware of that but it slipped my mind completely, thanks for pointing it out. The only issue arises if aga doesn't have formic acid, in
which case it might be simpler using a balloon of elemental hydrogen.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I do not have formic acid.
There are plenty of ants hereabouts, however i will not collect and distill them.
I suppose it could be purchased though.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
More references on Pd/C hydrogenations with ammonium formate as H donor:
http://www.organic-chemistry.org/abstracts/literature/250.sh...
https://www.erowid.org/archive/rhodium/chemistry/cth.af.revi...
https://www.ocf.berkeley.edu/~jmlvll/lab-reports/chemoselect...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | I do not have formic acid.
There are plenty of ants hereabouts, however i will not collect and distill them.
I suppose it could be purchased though. |
Ammonium formate is easypeasy to obtain.
[Edited on 5-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. It's going a bit crazy, so needs some parameters.
There is a lot of glassware, a single stage vac pump, some reagents (IOC + a few OC) on hand.
Locally available materials include plumbing supplies, so steel tubing/fittings are fine.
Translate Ammonium Formate into Spanish, please, then try to find it.
It could be Ant Powder, Artichoke Booster, or Alfalfa Stimulant (it is that random).
200 euros is about the limit for a single experiment at my level of learning.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Conversion 1 to 2 is treatment with GAA, no sweat.
After separation/mild clean up of 2, the conversion of 2 to 3 is simple alkaline de-esterification, probably in MeOH. Plenty, plenty plenty recipes to
emulate, no sweat.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | OK. It's going a bit crazy, so needs some parameters.
There is a lot of glassware, a single stage vac pump, some reagents (IOC + a few OC) on hand.
Locally available materials include plumbing supplies, so steel tubing/fittings are fine.
Translate Ammonium Formate into Spanish, please, then try to find it.
It could be Ant Powder, Artichoke Booster, or Alfalfa Stimulant (it is that random).
200 euros is about the limit for a single experiment at my level of learning. |
200 Euros? You're laughing, no sweat.
NH4 formate: eBay I think. It has some uses and should be very cheap.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yes. Ebay. 250g is now on it's way from Poland.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You don't hang about much... 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Can't.
If i delay even 1 day, the postage time to Spain is so long that i forget what the item was intended for.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Can't.
If i delay even 1 day, the postage time to Spain is so long that i forget what the item was intended for. |
Have you looked for alpha-pinene? There's a US Amazonner...
Anyway, let's continue most procurement chatter by U2U: no point cluttering up this thread with mundanities...
[Edited on 6-12-2015 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by aga  | Quote: Originally posted by Darkstar  | @aga
I hope you've been paying attention this semester, because I've written your final exam! The test is 25 questions plus an extra credit question. Just
say the word when you're ready and I'll post it.
|
Please !
Can't say i'm 'ready' per-se, however fire away. |
Don't worry, the exam is actually extremely easy for the most part. There is one question in particular (it's one of the first five) that might be a
little tricky, though. The exam consists of 25 questions and two optional extra credit questions (added a second one). For questions 1-20 and the
second extra credit problem, you can write the answers in the body of your reply post (just type "red" or "blue," "nucleophile" or "electrophile"
etc); for questions 21-25 and the first extra credit problem, you will need to use either ChemSketch or MS Paint-in-the-ass.
While the exam shouldn't take very long to complete, please feel free to do it at your own pace. There's no need to rush. All I ask is that you try to
do as much of it as you can without looking up the answers, particularly the last five questions. These questions are more or less the "real" test, so
to speak. The last mechanism (question 25) is one that blogfast recently covered, by the way. Also, don't get too hung up on the extra credit problems
if you're having trouble figuring them out, especially the first one. I did give a ton of hints for the second one, though, so it'll probably be the
easier one to figure out. (I practically gave you the answers!)
Anyway, here's the test. I took the liberty of typing your name in the blank for you:
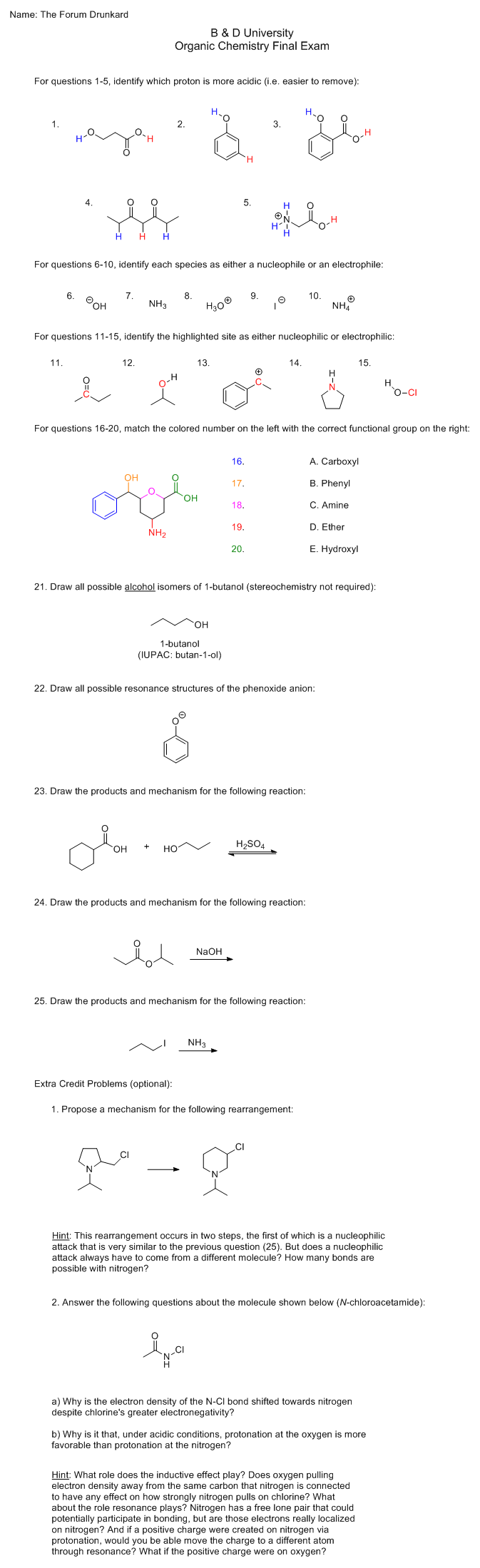
[Edited on 12-6-2015 by Darkstar]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Smashing, Darkstar. Will be taking that test myself. Forever young! 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
aga's OC Final Exam Answers Part 1
1 Red
2 Blue
3 Red
4 Red
5 Blue
Used the 'ARIO' rule i found on utoob.
6 Nucleophile
7 Electrophile
8 Electrophile
9 Nucleophile
10 Electrophile
11 Electrophilic
12 Nucleophilic
13 Electrophilic
14 Nucleophilic
15 Electrophilic
16 B. Phenyl
17 E. Hydroxyl
18 D. Ether*
19 C. Amine
20 A. Carboxyl*
* Had those two backwards before i checked
Drawn answers to follow (hopefully before xmas).
Takes some time to work stuff out, then longer to draw them !
[Edited on 6-12-2015 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
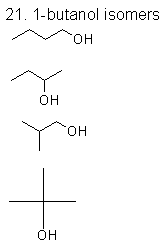
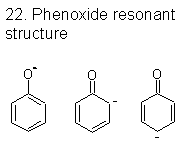
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
This was totally stolen from your example on page 15 of this thread, just with a phenyl ring and an extra wiggly bit on the ethanol.
[Edited on 6-12-2015 by aga]
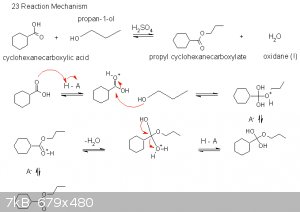
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Sorry, but you only went and used the so caligraphically wrong types of double arrows in that last answer. We're very pedantic about these
things here at B&D. Fail!
(just kidding)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Are you drunk ?
If not, try harder !
Look again.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not sure about this one at all.
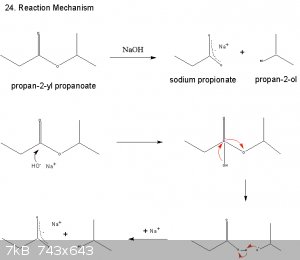
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
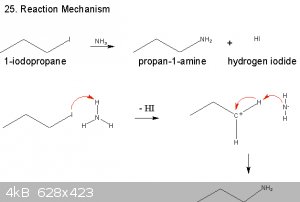
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
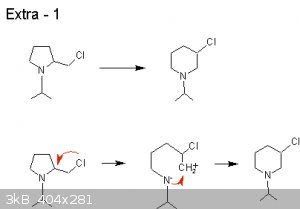
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Extra #2
a)
The electron density is more concentrated around the Nitrogen due to :-
1 Resonance
2 Small difference in electronegativity between N and Cl
3 the presence of the electron density pulled away from the H bound to the N
4. Cl has nowhere to pull electron density from apart from the N, pulling stronger on the resonance electrons through the inductive effect.
b)
Do not really know - it seems that a) negates the answer i first thought of for b).
Stab in the dark #1 :-
The C attached to O and N gets strongly +ve, allowing the A- to a come in with a nucleophilic attack, pushing electron density up to the O, allowing
the H+ to perform an electrophilic attack on the O.
S.I.T.D. #2 :-
The N is better protected in terms of physical shielding from the Cl/C/H and the pull of the Cl imparts a less -ve charge on it than that of the O.
S.I.T.D #3:-
the incoming H+ approaching the O tips the balance of the resonant electron density to be more strongly around the O, allowing the H-O bond to form.
Phenolpthalein product finally got recovered.
Yield = 0.31g = 9.2 % based on phenol (pitiful).
[Edited on 7-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Phenolpthalein product finally got recovered.
Yield = 0.31g = 9.2 % based on phenol (pitiful).
[Edited on 7-12-2015 by aga][/rquote]
Due to the filtering mishap, I guess? Ah well, out it down to experience and move on, I guess...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yes, it was over-vacuum that caused the filter to pop.
Similar to Nile Red's result, this is a beige powder, so also contaminated and not pure phenolpthalein.
I suspect that the workup could be improved in that process.
[Edited on 7-12-2015 by aga]
|
|
|
| Pages:
1
..
25
26
27
28
29
..
33 |