| Pages:
1
..
27
28
29
30
31
..
33 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes, all of them form bivalent hydrides(H2X) and have two lone electron pairs on the X. They're all Lewis bases, to varying degrees.
[Edited on 11-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
And now I have to add the following.
Hybridisation also depends on atom size and difference in electronegativity, see e.g. the highly stable SF6. It's shape can be found here.
The hybridisation is sp<sup>3</sup>d<sup>2</sup>, six equivalent half-filled AOs, ready for bonding to the F atoms.
[Edited on 11-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Also:
For double bonds in C-C bonds, both C atoms hybridise to sp<sup>2</sup> and the p<sub>z</sub> isn't involved. The
sp<sup>2</sup> AOs bond with each other and the H atoms (σ bonds), the two p<sub>z</sub> form a π bond (above and below the
C-C σ bond).
Will add a figure (not to scale):
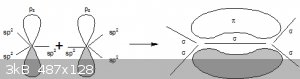
All half-filled sp<sup>2</sup> AOs and σ bonds in the xy plane. Two ungerate half filled
p<sub>z</sub> (z axis) AOs form a π bond (above and below the C-C σ bond).
Note: no hydrogen atoms shown.
[Edited on 11-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Enlightenment has finally occurred.
I think i finally Get It.
Well taught teach !
Now to see if i can apply the teachings ...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Assignment: draw a triple bond: 1 σ + 2 π bonds. The hybridisation is sp.
Take your time.
[Edited on 11-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Feck
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Tried in ChemSketch and got nowhere.
Ditto MS Pain.
Best i could manage was in Fireworks :-

Blue is the sigma bond, green one pi bond orbital, magenta/red the other pi orbital, with magenta behind, red in front.
[Edited on 12-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
We're like sips passing in the night.
That's correcto!
I'll show you mine.
[Edited on 12-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sops pissing to the right ?
Stop mexing your mitafors.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ships. LOL.
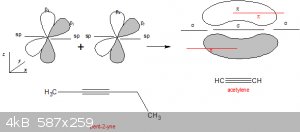
I had a bit of trouble with it in ChemSketch too.
Ignore the acetylene and pent-2-yne for a minute.
My second pair of π lobes are in (parallel to) the xy plane and painted as red solid lines. So your structure is correct.
With one H on each of the two sp orbitals we get acetylene (IUPAC: ethyne), which is an interesting case. Due two the high electron density between
the to C, both H are very slightly acidic (but less than ethanol, e.g.) and salts of it exist, in particular calcium carbide,
CaC<sub>2</sub>. With water that generates acetylene because the acetylene is such a weak acid that even water displaces it.
Another thing about alkynes is that the triple bond imparts on the molecule some linearity not seen in alkanes, see the case of pent-2-yne,
where the C atoms 1,2,3 and 4 are all on one line! Compare to the 'squiggly' structure of pentane, e.g.
And talking about double/triple bonds, I'm going to present a few more exercises here, shortly.
[Edited on 13-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Using the electrophilic addition mechanism to alkenes and Markovnikov's Rule, predict the reaction products of the following additions:
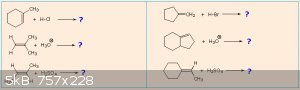
[Edited on 12-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not sure about any of these, especially the last one.
It just looked stupid so it got rearranged to look better.
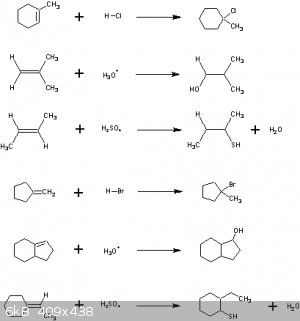
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The answers:
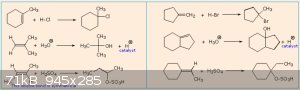
Quite a few errors there. Not quite sure why.
The ones with H2SO4 are perhaps understandably harder because you have to realise that HSO<sub>4</sub><sup>-</sup> acts here
as a Lewis base and latches on to the carbocation. Will add a diagram tomorrow.
Markovnikov says that the carbocation is formed on the C-atom that is already the most connected to other C-atoms.
In order of stability: primary carbocation < secondary carbocation < tertiary carbocation.
[Edited on 15-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yes, sorry.
#5 was just idiocy and not being able to count to 4.
Seeing the mechanisms for #2 and #3 would be very helpful.
I think i've hit my limit on how much can be absorbed in a given time.
Certainly need to revise the past several lessons again (a few times).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
#5 was just idiocy and not being able to count to 4.
Seeing the mechanisms for #2 and #3 would be very helpful.
I think i've hit my limit on how much can be absorbed in a given time.
Certainly need to revise the past several lessons again (a few times). |
#2:
Note that H2SO4 === > H+ + HSO4(-)
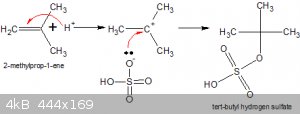
Although the hydrogen sulphate ion (bisulphate ion) is a Bronsted Lowry acid (quite a strong one too!) it has unbonded electron pairs on the O atom,
one of which bonds to the hard Lewis acid that is the t-butyl carbonium cation. That bisulphate ion is negatively charged helps, of course.
The resulting structure is itself a strong acid by virtue of that dangling -OH group.
In ChemSketch the structure can be easily drawn as follows. Draw your C structure. Add an O atom where you want the sulphate to be. Choose
HSO<sub>3</sub> (right hand side of structure menu) and add it to the O.
A well known acid in this category is the following:
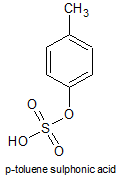
It's a strong, non-oxidising acid, often used in OC.
************
Why don't we build in a pause and you can work on your empirical dissertation: the conversion of alpha-pinene to alpha-terpineol (and beyond)? 
I've found another potentially doable reaction with alpha-pinene: the preparation of bornyl chloride, so the money spent on a bottle of high
quality turpentine would be well spent! 
[Edited on 15-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
A Pause before further enlightment rains down would be appreciated.
Some practical would be welcome !
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | A Pause before further enlightment rains down would be appreciated.
Some practical would be welcome ! |
Agreed. 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
'Blast from the past':
http://www.falstad.com/qmmo/
To see actual calculated versions of the most common molecular orbitals, use this latest version version of the Java applet. They are for the
H2<sup>+</sup> molecule (no inter-electronic repulsions).
Only works in IE (NOT Chrome) and you'll probably have to gratis upgrade your Java version.
A π (ungerate) 2px molecular orbital:

[Edited on 20-12-2015 by blogfast25]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Fugitive Fermions
I explain quantum tunneling to my younger brother.
He says:
You mean, El Chapo electrons?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Each half is remarkably similar in shape to a Haemogoblin-containing cell.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Blog the structure you show is not p-TsOH. p-TsOH forms via electrophilic aromatic substitution as the active electrophile is HSO3/SO3 where the
sulphur is attacked by the C=C in the arene.
however the below mechanism would in practice be completed using oleum, rather than H2SO4 as with toluene. This is as the methyl group is electron
donating which activates the ring system.
source: http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch12/ch12-4...
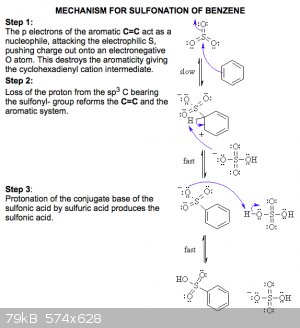
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by HeYBrO  | Blog the structure you show is not p-TsOH. p-TsOH forms via electrophilic aromatic substitution as the active electrophile is HSO3/SO3 where the
sulphur is attacked by the C=C in the arene.
|
Ok, thanks.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
I gave the correction not so much for you but just so no one becomes confused with your structure, hence gave an explanation of the mechanism. great
thread btw
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by HeYBrO  | | I gave the correction not so much for you but just so no one becomes confused with your structure, hence gave an explanation of the mechanism. great
thread btw |
No worries.
That mechanism you showed seems for the sulphonation of benzene with oleum, though, as it starts with an attack on the π-ring by SO3, not
by H<sup>+</sup>.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by HeYBrO  | | I gave the correction not so much for you but just so no one becomes confused with your structure, hence gave an explanation of the mechanism. great
thread btw |
No worries.
That mechanism you showed seems for the sulphonation of benzene with oleum, though, as it starts with an attack on the π-ring by SO3, not
by H<sup>+</sup>. |
I am aware that it is benzene and oleum in the mechanism and hence stated so in my original post. Further, what does H<sup>+</sup> have to
do with sulphonation/what i posted? it is not required for sulphonation of aromatic rings in what i have posted (toluene and benzene). I don't recall
of any sulphonation in this context that has H<sup>+</sup> as the active electrophile.
edit: i must be misunderstanding what you mean.. Could you please explain?
[Edited on 18-1-2016 by HeYBrO]
|
|
|
| Pages:
1
..
27
28
29
30
31
..
33 |