| Pages:
1
2 |
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Low melting salt mixtures
I need appr 4kg of a low melting salt mixture for a tempering bath. I'd like to get below 200°C, which in it self isn't that hard, the challenge is
availability of chemicals. Basically I have sodium and calcium nitrate, and assuming the reduction with lead works sodium nitrite as well.
I've been searching like mad, but most mixtures uses potassium nitrate and even more exotic salts like lithium. Does anybody have a good source for
useful compositions?
One source claims 145°C for 50/50 NaNO3/NO2 while other sources say 220+, so it's hard to know where to start.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Calcium nitrate tetrahydrate melts at around 40 oC, and loses water at 132 oC.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
It loses water and becomes a solid again. Which decomposes at ~500C before melting.
Have a look at this: http://www.crct.polymtl.ca/fact/documentation/FTsalt/FTsalt_...
A LiNO3/NaNO3 eutectic can get you to 195C. KNO3/NaNO3 eutectic will get you to 223C.
LiNO3/KNO3 is very low-melting at 125C.
Of course, LiNO3 is fairly expensive and that website doesn't have nitrites included.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have used the tin/bismuth eutectic (mp =139°C) but I needed less than a kg.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
To elaborate a bit, I need a annealing/tempering-bath that can operate in the <200-400+°C range for hardening and tempering steel. And as I need
at least 2l (4-5kg in the case of nitrate salts) I need something cheap and available. The Bi/Pb/Sn-alloys are interesting, but the density would make
steel float. And then there's the fact that I would need 20kg...
UC235: Wonderful source, but sadly it does not contain any useful candidates. Unfortunately I am completely out of KNO3, as most mixtures seems to
rely on that. And KNO3 has become increasingly hard to get in recent years. I would prefer to avoid the nitrites as well (suspected carcinogenic and
teratogenic), but compared to the dangers of hot molten salts I'm not sure if that is any real issue. And as I have both sodium nitrate and lead it is
at least something I could make.
Best candidate so far is 46:24:30 K-Na-Ca nitrate with a listed mP of 160 C.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
I've been searching for low melting NaCl mixtures so I can make sodium metal and came across this excellent compilation of eutectic mixtures:
http://www.nist.gov/data/nsrds/NSRDS-NBS-61-1.pdf
There's a lot more nitrate mixtures that you could try.
AlCl3 mixtures also have very low melting points but getting the anhydrous salt is pretty hard. Putting Al in hydrochloric acid will produce the
hydrate, which according to Wikipedia, cannot be heated to get the anhydride.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Thank you sir, this was exactly what I was looking for.
I'm focusing on nitrates only as I know these are used for heat treatment. And I have been advised against chlorides by a metallurgist, and while he
didn't give any detailed reasons I have no reason to doubt his expertise.
What I was hoping for was a Ca/Na NO3 mix below 200C, but no such luck. At 31 mol% it has a mp of 214C, which isn't that bad really.
Edit: Dang, I thought I found a good one at 154C, but that was with Ca(NO2)2. So 214°C it is, I can live with that as long as I don't have to make
any chemicals.
[Edited on 14-7-15 by Fulmen]
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Huh! I threw together a quick test, but got nowhere. The mix was listed as Ca(NO3)2-NaNO3, 31 mol%, which I assumed was 31 m% NaNO3. Could it be 31%
Ca?
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Generally the material with the highest concentration is listed first. So most likely sodium is 31%.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=10529#...
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Thank you, I'll have a look and see if there is anything useful there as well.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
AlbinoMoose308
Harmless

Posts: 4
Registered: 27-1-2015
Location: TN, USA
Member Is Offline
Mood: No Mood
|
|
I took a look at that PDF and it seems the first concentration is for the first of the two components. For example the concentration of #86 is listed
as 8.1-87.6-4.3. You need 31% Ca(NO3)2.
Acquiring a lab is difficult when you're dead-set on not spending a penny
Chlorine trifluoride should be officially designated "liquid fire"
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Now there is a Franklyn that i respect. Nice one.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
| Quote: | | Chlorine trifluoride should be officially designated "liquid fire" |
I just looked that up, and fail to see how it exists.
F and Cl each have 7 electrons in their outers, so are looking for just 1.
Surely F & Cl should form FCl and be rather pleased with themselves.
How does it get to ClF3 ?
[Edited on 22-7-2015 by aga]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Fluorine is so electronegative, it even forms ClF5!
https://en.wikipedia.org/wiki/Chlorine_pentafluoride
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Much in the same way the other +1, +3, +5 and +7 Oxidation Number chlorine compounds exist: see hypochlorites, chlorates and perchlorates.
[Edited on 23-7-2015 by blogfast25]
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Albino Moose: Logic dictates it has to be either one or the other, right? I'll try the reverse proportions when I feel like working again.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Fulmen, what part of the world are you in?
Are you heat treating steel tooling, knives, or ??? Any particular alloys and profiles you could disclose?
There's over 500lb. of Potassium nitrate, plus good quantities of all the other common nitrates on site here, and one of my crew is an amateur
blacksmith.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Here's a useful table for mixed nitrates eutectics made anhydrous by heating
http://www.sciencemadness.org/talk/viewthread.php?tid=4457&a...
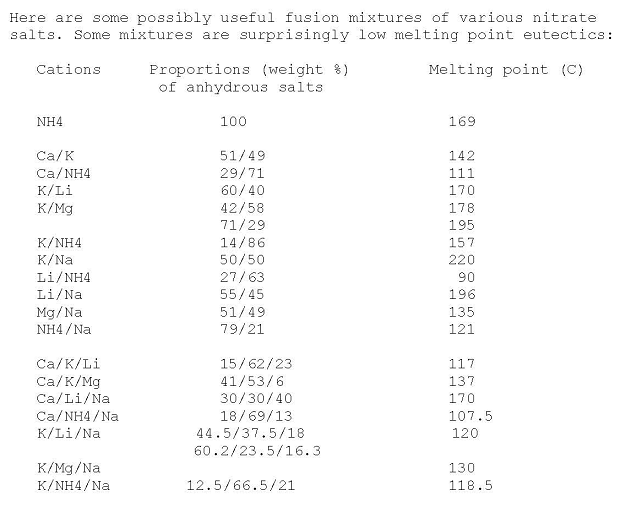
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
That Ca/NH4/Na nitrate 18/69/13 eutectic mixture melting @ 107 C. sounds quite interesting for other things than an annealing bath... Given that
pyrotechnic reactions tend to start in earnest at the temperature where the oxidizer becomes molten .
Unfortunately, it would likely be rather hygroscopic.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
This project must be put on hold for now, most of my access to proper tooling is gone so I might not work too much on machining. But I'll keep it
alive on the back burner, scouting for what I need.
[Edited on 24-7-15 by Fulmen]
We're not banging rocks together here. We know how to put a man back together.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ancient chinese proverb :-
Make Rocket in Summer.
Fart in tube.
Seal Tube tight.
No more atmosphere any self-respecting chemical want to absorb.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Eutectic nitrates sounds like a good way to improve casting properties of sugar propellants. The K/HN4-mix is especially interesting if it improves
burn rate over straight NH4, although I have seen tests (20 years ago) that showed signs of reaction with the fuel when molten (liberation of gas).
Don't remember which fuel they tested though, could have been charcoal.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
59 wt. KOH and 41 wt. % NaOH eutectic at 170°C. See US 20050072837 A1 patent application for this and others. HIGHLY dangerous of course!
|
|
|
MeshPL
Hazard to Others
  
Posts: 329
Registered: 20-4-2015
Location: Universe
Member Is Offline
Mood: No Mood
|
|
In my "chemistry tables" book I've found the following compositions, none of them being nitrates:
50% ZnCl2 + 50% KCl m.p=.230C
60% ZnCl2 + 20% NaCl + 20% KCl m.p.=203C
67% AlCl3 + 33% KCl m.p.=128C
And finally unbelievable:
60% AlCl3 + 26% NaCl + 14% KCl m.p.=94C (!)
I assume zinc and aluminium chlorides are not hydrates here.
Note that anhydrous zinc and aluminium chlorides cannot be prepared with aqueous HCl!
|
|
|
| Pages:
1
2 |