YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
Wool Dying
No I havent finaly lost my mind, I`m doing this for my dear old Mother, she`s into carding and spinning wool and making things, and since she knows I
have plenty of chems, she asked me to synth a few dyes, she`s 60 the end of this year and Finaly Trusts me with chems (I`m 40 next year!)  yeah it took a long a time! yeah it took a long a time!
so anyway, I have 15 different colors already but need to concentrate more on Reds, Purples/Violets, a few more Blues and some grey shades.
thus far all reactions have been Water based and I`m still open to more!
and also Cold water too.
denatured alc (methylated spririts) I haven`t tried yet, so fire away.
I`m basicly after novel colors that are simple to make and idealy metal salt based, or at least easily obtainable orgo based.
WHEN (he says HAHA) I get my cam server up and running again, I`ll send a pic of the samples I have, there really are some Fantastic colors to be had!
silver nitrate and gold chloride make a few less interesting ones than I`de hoped for, but Chlorophil or tartrazine are good, as is potassium
hexacyano ferrate and feSO4
the latter looks a bit like belly button fluff color, and blends in perfectly with my faded levis.
a Very Odd thing though KMnO4 soln makes the wool jet black!
not a deep X color that LOOKS black, but 100% Pure black, even under a microscope.
however it Does denature the wool a little and make it brittle, so any good Black ideas would be welcome too.
any workable ideas (chems permitting) Are welcome 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
If colour chemistry is your thing, than the sky is the limit. The chemical industry evolved out of the need to produce synthetic dyes. Until a few
decades ago, the chemistry of dye synthesis was an established part of the organic chemistry course. Azobenzenes formed an important category of
compounds. A few syntheses can be found on Lambdasyn: http://www.thorsten-hoffmann.de/htm/news.htm
But what I suggest to do is the following: go to a university library and locate the old and dusty books. Chances are very high you turn up with
something. If you're lucky, you can find books that are nothing more than lists of dyes with colour examples, references to their synthesis (usually
patents), ... But I admit these books are a bit harder to find.
damnant quod non intelligunt
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
A good book that may get you started is the Sigma-Aldrich Handbook of Stains, Dyes and Indicators by Floyd J. Green. It lists many synthetic dyes and
includes basic chemical references: CAS, molecular weight, appearance, UV (including molecule drawing), solubility in several solvents, appearance and
last but not least, the Sigma catalogue number! Furthermore, it gives a few applications. For instance Naphthol Yellow S:
Naphthol Yellow S
Acid Yellow 1
8-Hydroxy-5,7-dinitro-2-naphthalenesulfonic acid, disodium salt
[...]
Industrial applications for Naphthol Yellow S include the dyeing of wool, leather, furs and paper, coloring soap, the formation of heavy-metal lakes
for use as pigments in printing inks and for coloring rubber.
Also, many of these compounds are not that difficult to synthesize, but a real disadvantage may be that you're going to paint the whole
neighbourhood and not just your wool 
damnant quod non intelligunt
|
|
|
triphenylphosphineoxide
Harmless

Posts: 30
Registered: 18-5-2006
Location: East Aus
Member Is Offline
Mood: No Mood
|
|
What type of mordant are you intending to use (if any)?
Precoating the wool opens up a lot more alternatives.
Off to search for dye recipes and natural products, I have this information somewhere.
|
|
|
triphenylphosphineoxide
Harmless

Posts: 30
Registered: 18-5-2006
Location: East Aus
Member Is Offline
Mood: No Mood
|
|
Still searching for the mother load but have found my partial notes on plant extract dyes.
To start with general effects of mordants:
Chrome :warms colours esp. reds and yellows
Iron: increases grey of colour
Recipe for Chrome mordant
28g Pottassium dichromate, 21g cream of tartar in 16 L water per Kg of wool
This site has recipes for Iorn, Alum and Copper Mordants, the dyes stuffs listed here also provide a lot of Greys but access to them may be a problem
in the UK.
http://farrer.csu.edu.au/ASGAP/APOL8/dec97-6.html
As for red dyes Carrots create an orange that can be darkened using ferrous sulphate
and from memory coffee and red onion skins produce a red almost brown dye I've only tried iron with these so possibly a different mordant would
produce a stronger red.
All of the above dyes were simply extracted with boiling water and left to steep for 24 hours.
Net searching just found purple and a black
Inner bark of Red Maple with Iron Mordant = Purple
Blackberry mordant ?? = Purple
Sumac Leaves mordant unspecified but probably Iron =Black
[Edited on 28-7-2006 by triphenylphosphineoxide]
http://www.pioneerthinking.com/naturaldyes.htmlv
More colour ideas
http://aic.stanford.edu/jaic/articles/jaic21-02-003_3.html
More information than is usefull on dye/mordant interactions
[Edited on 28-7-2006 by triphenylphosphineoxide]
|
|
|
Vitus_Verdegast
Hazard to Others
  
Posts: 297
Registered: 5-12-2004
Location: Ottoman Empire
Member Is Offline
Mood: tea time
|
|
You have not missed these ?
http://www.sciencemadness.org/library/books/the_chemistry_of...
http://www.sciencemadness.org/library/books/the_dyeing_indus...
http://www.sciencemadness.org/library/books/dyes_classified_...
http://www.sciencemadness.org/library/books/fundamental_proc...
Sic transit gloria mundi
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
Thanks! that`s fantastic 
if nothing else, it`s certainly a Foot In the Door so to speak 
and yes, I was looking towards Chrome (or metal salt dyes), the Copper you mentioned is great, I actualy did that one, and made the most fantastic
Grey/Green color you can imagine, in fact when wet, it looked like wire wool, I soaked it in weak copper sulphate overnight and then added Ammonia,
making tetramine copper (II) sulphate, although what actualy took place in the wool fibers I have no idea, as it wasn`t the lovely Blue color of the
soln.
keep em comming 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
triphenylphosphineoxide
Harmless

Posts: 30
Registered: 18-5-2006
Location: East Aus
Member Is Offline
Mood: No Mood
|
|
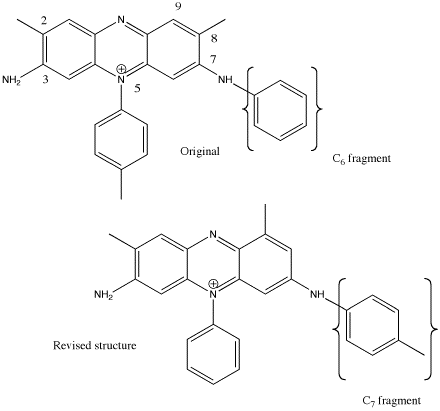
For historical value and purple why not try Perkin's Mauve
The ingredients are not exactly OTC but the synthesis is not that difficult.
To produce 4 mg of Mauve (about enough for a tie if not diluted)
Add 104ul aniline
120ul o-toluidine
122mg p-toluidine
1.2ml 2N sulphuric acid
to 5ml water
Stirand heat gently to dissolve.
add 60mg Potassium dichromate
Stir for two hours
Solutrion is now a rich purple.
Filter with gentle vacuum and wash with dstilled water untill filtrate runs clear.
Dry at 110 C for 30 minutes
Wash with ether untill clear
Dry for 10 min at 110 C
Wash with 25% methanol/Water untill liquid runs clear do not over wash at this stage.
Evaporate last filtrate to dryness.
Dissolve in methanol untill desired shade is achieved.
This does not require a mordant for Silk or Wool but cotton must be pre treated with tannic acid.
|
|
|