hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Why No Thiosulfate for Organic Reduction
I keep seeing threads describing the use of somewhat "exotic" chemicals for organic reductions. My limiited background in chemistry is almost
entirely non-organic, so when I think of "reduction" the first things that pop into my mind are thiosulfates and/or sulfites. Yet I don't think I've
ever seen these mentioned as reducing agents for organic reactions. Why is this? Maybe because of poor soluability in organic compounds?
Hodges
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
I don't think S2O3 is a strong enough reducing agent.
The ones I think are typical for organic reductions, e.g. NaBH4, LAH, alkali metals in NH3, RP/I2, are much stronger than thiosulfate or sulfite.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
For an example see
http://www.worldwideschool.org/library/books/sci/chemistry/O...
They are not strong enough for many desired reductions, in some cases can react in other ways, or otherwise are not compatible with needed conditions.
As an example, while many quinones' reduction potential is in range of sulfite, SO3H is added to the quinone rather than performing a simple
reduction.
|
|
|
len
Harmless

Posts: 35
Registered: 17-7-2006
Location: Australia
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by pantone159
I don't think S2O3 is a strong enough reducing agent.
The ones I think are typical for organic reductions, e.g. NaBH4, LAH, alkali metals in NH3, RP/I2, are much stronger than thiosulfate or sulfite.
|
Correct me if Im wrong, in the P/I system the I- does the reduction and thats not very strong is it? And on a separate but related question why is the
illegal reaction done with only this system?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Iodine is working, in effect, as a carrier
4I2 + 2 P => 3 PI3
PI3 + 3 H2O => 3 HI + H3PO3
PI3 + 3 ROH => 3 RI + H3PO3
HI + RI => RH + I2 ( recycles in 1st eq.)
So it's HI doing the direct reducing on the alkyl iodide, and phosphorus reducing iodine to the HI.
hmmm ... part of that here http://www.chemguide.co.uk/organicprops/alcohols/halogen.htm...
As for why it's used by cookers, it can be done in simple apparatus with fairly easy work-up - just H3PO3 and HI to contend with. Iodine and
phosphorus are chemicals you would almost bump into walking about, not that long ago, as opposed to alternatives. And the alternatives are a bit more
complex, generally not one pot operations like P + I, or lower yield.
The popularity of the reaction has made it tougher for those who have other uses for iodine or red phosphorus, especially those residing in regions
where highly regulated products of such a reaction are popular and have become the new Demon Rum of the press.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
@not_important
| Quote: |
HI + RI => RH + I2 ( recycles in 1st eq.)
|
What is the mechanism and driving force for the above reaction? I don't think I have ever seen anything like it in my reading or organic courses.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
For some reason, CRC does not list hydrides (or HI for that matter) in their electrochemical series.
Hodges
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Sodium thiosulfate is useful for reductive aminations, but if I am not mistaken the required conditions, solvents and yields are such that the system
is very unattractive compared to activated aluminium (which in addition has the advantage to work nicely on substrates which are not excessivly
purified).
/ORG
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I don't know the actual reason the reaction goes, but I will speculate.
Hydrogen iodide has a near zero heat of formation, it easily decomposses back to H2 and I2. Similarly the I-I bound is fairly weak, the C-I bound
only modertly strong, while the C-H bond is pretty strong. Ah
bond deltaH0
------ ---------
H-I 71
C-I 51
I-I 36
C-H 99
So it's a push energetically between the two sides of the reaction. It would seem that simply having an excess of HI could drive the reaction. In
the P/I reduction the frred iodine is constantly being converted to PI3 and then HI, which definately unbalances equilibrium.
The reaction was used in the early days of carbohydrate chemistry. Cyanide would be added to the carbonyl in a carbohydrate, converting it to a
hydroxy-nitrile. This would have all its HO- reduced to hydrogens using HI. The resulting nitrile would then be identified by comparing it to known
nitriles/carboxylic acids, which reveiled the structure of the carbon skeleton and where the carbonyl group had been.
Couple of online references to the reaction:
http://www.arkat-usa.org/ARKIVOC/JOURNAL_CONTENT/manuscripts...
http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1934/56/i03/...
|
|
|
len
Harmless

Posts: 35
Registered: 17-7-2006
Location: Australia
Member Is Offline
Mood: No Mood
|
|
OK. I think I see.
RI + I- + H+ -> RH + I2
looks like the key here. This is the actual reduction. All other reactions in the chain would happen with Br2 say. But this one I dont believe
would. The P/I system has the effect of turning I2 into a catalyst enabling P to reduce the alcohol.
HI is one of the strongest acids known. So since water is present there would hardly be any actual HI, just the I- and H+ ions. Since I- actually
reduces the RI, I believe RI->RH could be done with just about any other reducing agent, its getting it to form which is the trick, and here the P
is paramount.
In fact I am sure this reaction would not work with any other halogen. Else an alkyl chloride formed with difficulty by say reacting an alcohol with
conc. HCl would, on the addition of further HCL turn into an alkane and liberate Cl2, which is nonsense.
PS since I2 is just the catalyst restricting it should have no effect. Its recoverable.
[Edited on 30-7-2006 by len]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Yeah, at the end, assuming excess of red P, you should have all the iodine as HI and the used phosphorus as H3PO3/HPO(OH)2 - a useful reducing agent
on its own. Depending on what your organics are, after adding water you should either be able to extract them out with a solvent or add base and then
extract the organic; the aquous layer having either the free acids or their salts, and excess red P being filtered off. If as the sodium salts,
recovery might be doable by evaporting and extracting the NaI with acetone.
It was a very useful tool in those early days of organic chemistry, for the researcher there's a lot of alternatives with better control around now.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
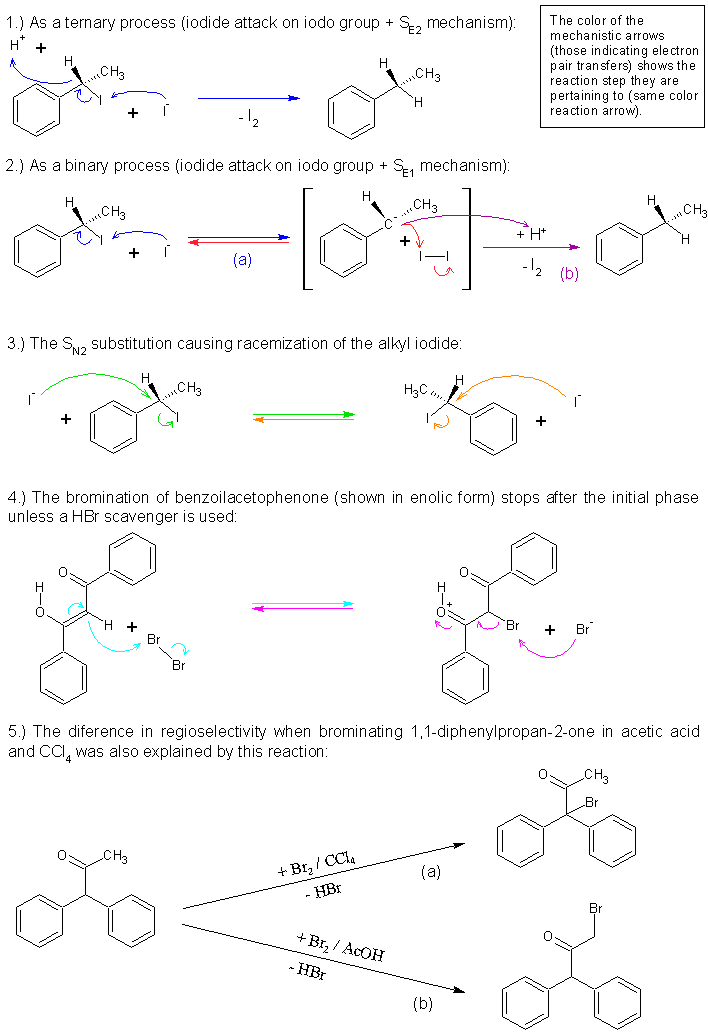
Mechanism
Len, your suppositions are in my opinion correct, but you surprise me. Wasn’t it you who in another tread once said: “As far as the reaction goes
its a piece of chemisty trivia”? Well, I’m glad you noticed it’s more than trivial. 
As far as the mechanism itself, I never saw its proposal depicted in any textbook and I do believe the fall in popularity (in legitimate labs) during
last five decades is the main reason. I’m sure Organikum will gladly explain the reason and chronology of the historical decline in its popularity -
if asked kindly enough. 
Attached is the mechanism as it would be classically assumed on the base of electron pair transfers theory. Note that this is just a hypothetical
mechanism and it does not say much on how atoms behave in real world. It is just a cognitive tool in order to help understanding some phenomena
(mostly only to lessen the chemists’ anxiety). It could well be depicted using single electron transfer (SET) theory in the first step, the iodide
attack (2.a). But that is not really important. Besides it is unknown to me if the process is binary (2.) or ternary (1.). But for what regards the
product conformation it does not have practical importance, since even if tertiary alkyl iodides which yield chiral compounds are used, one could not
get inversion or retention of configuration, but always the racemate due to the equilibrium of the iodide inversion reaction (3.), which is one
unavoidable reaction going on meanwhile. The reduction is the major practically irreversible one, but there are many reversible reactions going on as
well.
| Quote: | | All other reactions in the chain would happen with Br2 say. But this one I dont believe would. |
Hm, this assumption is not really general. There are known exceptional cases when this can happen with HBr/Br2 as well.
For example if you take the bromination of benzoylacetophenone (4.). It was found that the bromination of the activated methylene group starts
normally, but then it soon stops. This was explained on the base of the reversibility (the formed HBr reduces the bromoketone). The reaction indeed
proceeds if a base to scavenge HBr when it is formed is used.
Another case is the bromination of 1,1-diphenylpropan-2-one which was found to yield the kinetic product arising from bromination of the most
activated methylene group in CCl4 as solvent (5.a). But when the solvent is acetic acid the product obtained is from bromination of the least
activated methylene group (5.b). This was explained in that in non polar solvents (CCl4) hydrogen bromide is undissociated, so all is fine here (due
to lower solubility it also considerably bubbles out). But in acetic acid it is mostly dissociated and the HBr can reduce the kinetic product as it
forms. Thus yielding back Br2 which on turn can brominate again. Only the slower reaction on the least activated methylene group is irreversible
enough, so at the end only this product is obtained.
But as you can note these cases are exceptional as the halide is on a carbon neighboring to groups capable of stabilizing the carboanion (if the
mechanism is SE1) or accepting a bond (if the mechanism is SE2). The phenyl groups do have such stabilizing effects, but the ketone carbonyl groups
are even much better.
PS: I don’t agree in that I2 can be called a catalyst. That term is only reserved for materials having a catalytical role in the reaction
and I2 has none. On the contrary it can only inhibit the reaction (especially if the mechanism is SE1). I2 is only the end product of the HI
oxidation. What you probably meant is that it is recyclable (for example, by P, H3PO3…), but that is nearly not the same concept as
catalysis.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
The role of iodine is dependant on the substrate. With some substrates iodine is a true catalyst with others it is first a reactand serving as a
catalyst after the formation of an intermediate. The borders between this two ways are pretty fluid though, much depending on reaction conditions,
concentrations and solvents.
So the role of iodine is best called ambiguous. You won´t be able to fix it to the one or to the other side.
|
|
|