Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Organic geminal hydroperoxides as potential explosives
High explosives are metastable pure substances/mixtures capable (under the right stimuli) of releasing large quantity of energy in the form of heat,
light and work into the surrounding. Most practical high explosives in use today are formed from the element carbon, nitrogen, hydrogen and oxygen
(e.g., TNT, PETN, HMX, etc..). In recent years, high nitrogen content explosives become more an more important due to the fact that these molecules
are capable of storing large amount of potential energy (i.e. large positive heat of formations, HOF's). In high nitrogen content molecules, nitrogen
is bonded to another nitrogen atom through the formation of single and double covalent bonds and thus upon detonation the nitrogen forms the highly
stable, triple-bonded nitrogen molecule (N2) with the release of high quantity of energy.
On the other hand, other class of explosives didn't find their ways into practical applications due to their poor detonation performance and their
inherent instability. One example is peroxide-base explosives. Peroxide explosives (compounds containing -O-O- linkage) such as diacetone diperoxide
(DADP), triacetone triperoxide (TATP), hexamethylene triperoxide diamine (HMTD) are very popular among amateur chemist but these explosives possesses
low detonation performance and also high sensitivity toward impact, friction and electrostatic discharge. A recent study, shows that compounds
containing the -O-O- linkage like hydroperoxides (-O-O-H) may be useful as powerful explosives and acceptable sensitivity [1].
A series of geminal hydroperoxides were synthesized from their corresponding ketones or aldehydes (see Fig.1) and 30-50 wt% hydrogen peroxide
(H2O2) in the presence of molecular iodine (I2) or hydrogen chloride as catalyst:

The structure of the organic hydroperoxides along with their density and detonation performance is shown in Fig.2:
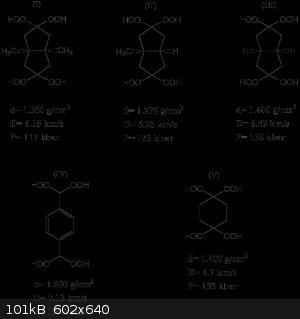
As can be seen in Fig. 2, these new hydroperoxides possess acceptable calculated detonation performance (i.e. calculation done with EXPLO 5
thermochemical code) ranging from 6.15-7.13 km/s for detonation velocity and 117-119 kbar to detonation pressure. Compound (IV) is even more
performant than TNT (D= 6.95 km/s, P= 190 kbar at d= 1.640 g/cm3 [2]).
It is also clear that all these hydroperoxides are denser and more powerful explosives than the well known TATP [1] (D= 5.3 km/s at d= 1.180
g/cm3). Moreover, the measured impact sensitivity (impact sensitivities were obtained on a BAM drophammer [1]) of compound (I-V) is less
than that of TATP (impact sensitivity= 1-3 Joules for compound (I-V) vs 0.18 Joules for TATP).
The electrostatic discharge (ESD) value of TATP is equal to 0.16 Joules. All the hydroperoxides (except compound (III)) have ESD> 0.2 Joules.
In conclusion, these new hydroperoxides shows that compounds containing the -O-O- explosophoric moiety can be used at least as powerful primary
explosives.
References:
[1] N.-D. H. Gamage; B. Stiasny; J. Stierstorfer; P. D. Martin; T. M. Klapotke; C. H. Winter, Less sensitive oxygen-rich organic peroxides
containing geminal hydroperoxy groups,Chem. Commun. 51 (2015) 13298-13300.
[2] C. L. Mader, Numerical modeling of explosives and propellants, CRC, Boca Rato (Fla.), 2008.
Dany.
[Edited on 27-8-2015 by Dany]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Just goes to show the book is not at all closed on peroxide compounds. Acetonitrile is not an over the counter
hardware store bought solvent so that will keep amateur dabblers form experimenting. The aryl variant , number ( IV ) could be further reacted with
ozone to obtain an aryl ozonide. That one should break records. It is entirely possible that hydroperoxides as these can form co-crystals or
clathrates. This would address the property shortcomings for practical applications. Compositional formulations with H2O2 might be worth
investigating.
www.sciencemadness.org/talk/viewthread.php?tid=3214
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@Dany,
Could you join the documents referenced?
Do those contain the HOF (heat of formation) or HOE (heat of explosion) of the compounds?
Got the doc, will read it.
[Edited on 29-8-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
|