deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Pourbaix diagram generating app
Pourbaix diagrams are very useful tools for the amateur inorganic experimentalist. A basic introduction is given here:
https://www.wou.edu/las/physci/ch412/pourbaix.htm
Recently I've discovered an app that constructs them for you FOR FREE and wanted to share this tool with the SM community.
https://www.materialsproject.org/#apps/pourbaixdiagram
Oxygen and hydrogen are included by default and you can pick from a whopping additional three elements simultaneously! You can also set the
concentrations, which is pretty neat and the unstable species formed are listed in the sidebar.
[Edited on 6-9-2015 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Exactly what I need at the moment. I haven't come across this diagram before but it is just the thing.
I have not been able to register at the site you gave. No email came to my email address. I will check the spam filters with IT tomorrow.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
You don't get an email AFAIK, mine just worked after registering.
[Edited on 6-9-2015 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Ok. I just got a popup message saying that an email had been sent... but nothin.
I signed in with my Google account and now I'm in.
What a site! I am really impressed. I can see this will be more than a little bit useful.
Quick question... I am interested in the Ni Cr system. (A student of mine has dissolved some nichrome wire and is playing with the solution formed.)
In one experiment she got a precipitate that as best as we could figure included nickel chromate. I don't see it on the diagram at all. So maybe I am reading it wrong or maybe I am missing something. Insight appreciated.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
It's there, just tried it, see the middle of the top of the diagram. It's only stable in a very narrow pH range and note concentrations I used.
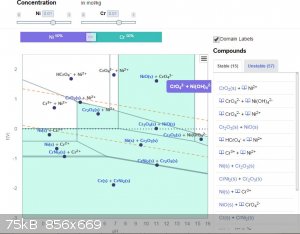
EDIT: Also to the left of this region, but then the chromate ion is protonated in solution.
[Edited on 6-9-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Use a pH meter to measure the electrode potential of your solution. You can then see exactly where in the diagram you're at after setting the
appropriate concentration.
See https://en.wikipedia.org/wiki/Reduction_potential
Don't forget to correct for the probe you're using with regard to the SHE potential.
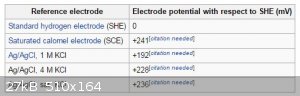
[Edited on 6-9-2015 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Well in that case, it looks like I was mistaken about the Nickel Chromate. I had it pegged as a precipitate.
Next question -- hep me read the diagram -- there are two sliders: one for the concentrations and the other changing the percentages. I am not quite
sure what that means. Surely altering one would alter the other but it seems to to work that way.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
And how would I use my pH meter for measuring the reduction potential? Isn't it measuring pH?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Depends on concentration, if you raise both Cr and Ni to 0.1M, you precipitate CrNiO4 in that centre top position. How neat is that!
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by deltaH  | | Depends on concentration, if you raise both Cr and Ni to 0.1M, you precipitate CrNiO4 in that centre top position. How neat is that!
|
Yeah. Cool. I spotted that.
This really is a great tool. Ideally it would be good for my student to be able to arrange conditions so that one metal was precipitated and the
other aqueous. She has been playing with the pH and adding oxidants and reducers but at this stage no clear patterns have emerged. Confounding the
issue is that most of the time her solutions take a while to equilibriate and so her first observation is not necessarily the correct one.
Anyway, this is exactly what is needed. But I do need to know how to drive the thing properly. So, how do I configure the sliders?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I believe most pH meters have a button that can toggle between the pH reading or the actual potential as measured by the probe (in mV) AFAIK.
Read the section entitled "Converting potentials between different types of reference electrodes" in the wiki reference I provided above for a step by
step way to convert for your probe.
[Edited on 6-9-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by j_sum1  | Quote: Originally posted by deltaH  | | Depends on concentration, if you raise both Cr and Ni to 0.1M, you precipitate CrNiO4 in that centre top position. How neat is that!
|
...Ideally it would be good for my student to be able to arrange conditions so that one metal was
precipitated and the other aqueous. She has been playing with the pH and adding oxidants and reducers but at this stage no clear patterns have
emerged. Confounding the issue is that most of the time her solutions take a while to equilibriate and so her first observation is not necessarily
the correct one.
Anyway, this is exactly what is needed. ... |
Yes this is what Pourbaix diagrams are meant for. From the basic trend I've seen of your system, Cr2O3 precipitates at lower pH and electrode
potential than NiO, so you should definitely be able to use it to first precipitate the one and then the other.
Not sure about the percentage slider, you would think that the concentration above overrides it? There is a help file/tutorial somewhere. I'm also a
learner, just discovered the app recently.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Ok. On the pH meter. The ones I have access to don't have that. Still, it looks like we will attain hat we want by maxing out the oxidation
potential. Excess H2O2 and away we go.
I will see if I can find out about those sliders.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
You also need an ORP probe, but the meter with a toggle function can be used to read it. Most people aren't aware that their pH meter can also measure
the REDOX potential of their solution!
[Edited on 6-9-2015 by deltaH]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Speaking of such things, does anyone here have experience with the PHREEQC package? I am still a little overwhelmed by it.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|