| Pages:
1
2 |
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Brainteaser #2
I made the statement that gases used in a cell generally require a catalyst, again... why?! This time a simple "for kinetic reasons" is too simple, I
want a deeper answer.
Clue: Ideal gas law.
[Edited on 7-9-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
OK, I'm tired and going to bed, so here's the answer not to keep you hanging...
We all know that chemical kinetics is a function of the concentration of reagents [to various powers]. For gases, one can re-arrange the ideal gas law
such:
PV = NRT
<=> N/V = P/RT
Now N/V is nothing else than a concentration... like moles per litre
so for a gas, the concentration is P/RT, so at atmospheric pressure and room temperature, this amounts too:
(1atm)/(0.08205736L atm K−1 mol−1)/298K = 0.04 mol/l
Pretty f@#$%ing dilute!
Now on top of this, sometimes the gases are non-polar, e.g. oxygen and hydrogen, and so they are also extremely insoluble in the liquid phase (oxygen
saturates in water in the ppm range).
No wonder the kinetics are slow with reagent concentrations like that and that's not even looking at the activation energies for dissociating
them(which are case specific).
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Sorry DeltaH I got lost reading stuff about electrochemistry.
Yes I know the concentration of gasses in water is quite low, henry's law, etc.
I know platnium is catalytic for many hydrogen processes, but no clue about oxygen. Inface oxygen likely poison's platinums catalytical influence on
those hydrogen proccesses.
Anyways, I am still completely stuck with my original question. How do I decide what electrodes can be used?
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
my original question. How do I decide what electrodes can be.
______________________________
Will it carry enough current?
Will it withstand carrying that current in that solution?( without failing or polluting)
Will it be able to assist your purpose? (If it needs something special)
(don't forget,).. is it a valve metal placed in the right polarity?
Then it works 
^^^ super simplified
______________________________
vvv less simplified, it really depends on what your doing.
- are you enacting a chemical reaction in one product by oxidation or reduction to make something new? like NaCl -> NaClO in a swimming pool
sanitation system.
- are you destructively oxidizing the media directly like some of the papers on ammonia, NO(x), SO2 abatement. Attachment: CLEAN NOx & SO2.pdf (639kB)
This file has been downloaded 307 times.
- or are you simply plating out metal.
lots a ways to go with electrochemical cells, hence the oversimplified answer or the detailed oriented need for set-up constraints.
------------------------------------------
I had intended to throw more time at this answer, but family fist, sorry if it seems half-assed. I love electrochem. recently been reading some
patents on the shor gold refining systems using ammonium chloride with nascent oxygen catalyst using gold scrap and a graphite rod as electrodes.
ceramic diaphragm pot as a separator. no nitrates or fumes was a nice draw. http://www.google.com/patents/US5009755
[Edited on 8-9-2015 by violet sin]
this paper is somewhat informative about MMO Vs. Pt & graphite ( top of pg 4) Attachment: water_star.pdf (1.1MB)
This file has been downloaded 1274 times. only 9pg's long with some decent reading like common mode of failure for MMO. it also had this, among other things to say.
"in order to provide the most suitable MMO for a given application the electrode manufacturer needs specific information about the intended use. For
example:- ... ..."
leading back to the simple/complicated way of answering will this metal work. special function or does it just have to not fail?
[Edited on 8-9-2015 by violet sin]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by smaerd  | Sorry DeltaH I got lost reading stuff about electrochemistry.
Yes I know the concentration of gasses in water is quite low, henry's law, etc.
I know platnium is catalytic for many hydrogen processes, but no clue about oxygen. Inface oxygen likely poison's platinums catalytical influence on
those hydrogen proccesses.
Anyways, I am still completely stuck with my original question. How do I decide what electrodes can be used?
|
Platinum is one of the better oxygen catalysts (relatively speaking) when also considering durability, so it's used in fuel cells on both sides. The
oxygen overpotential is quite high though compared to hydrogen's, so there's much research into improving that and also in making non-precious metal
catalysts with similar performance.
The wiki on 'overpotential' has a nifty table:
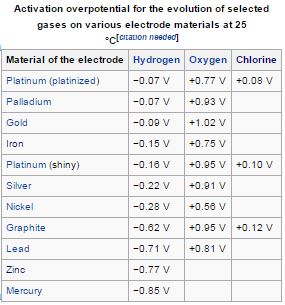
Source: https://en.wikipedia.org/wiki/Overpotential
I don't know much about oxygen poisoning the surface of platinum, I would have imagined that in a hydrogen atmosphere, this is very easily converted
to water and 'unpoisoned' 
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Quote: Originally posted by deltaH  |
I don't know much about oxygen poisoning the surface of platinum, I would have imagined that in a hydrogen atmosphere, this is very easily converted
to water and 'unpoisoned' 
|
Show's what I know about catalysis  . Thanks for putting up with me DeltaH. I
learned a good deal here. . Thanks for putting up with me DeltaH. I
learned a good deal here.
I also found the answer to my question! So I read through 3 of my electrosynthesis books in their relevent sections. Turns out electrode selection is
based on basically what sulaiman and DeltaH had said. It's typically experimentally determined based on corrosion, cost, etc. Although they do mention
that some electrodes favor or disfavor reactions at certain potential differences. So there's no clear cut way of doing this. Like any good question
it lead me to maybe 2 or 2000 more.
I'm still trying to wrap my head around the difference between the Cell potential verses the Electrode potential, and Open Circuit Potential (for
trickier systems).
It seems like the cell itself has an potential based on a given redox reaction pair (something I am trying to do). Then depending on what voltage I
apply to the physical electrodes I can either provide not enough electrode potential to instigate that reaction, enough to initiate the reaction, then
if I go over board the excess potential results in more current/faster rate of the reaction.
What I'm trying to figure out now is how can I tell if the electrodes I pick have interfering reactions. Like if I pick a silver electrode with KBr
then I have a rxn at 0.07V, but if I want to do a reaction at something like -0.10V the previous reaction would interfere with that happening(assuming
I am driving electrode potential negatively).
[Edited on 8-9-2015 by smaerd]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
| Quote: | | Thanks for putting up with me DeltaH. |
No putting up at all, I'm no expert in this, just sharing what I think I know 
| Quote: | | What I'm trying to figure out now is how can I tell if the electrodes I pick have interfering reactions. Like if I pick a silver electrode with KBr
then I have a rxn at 0.07V, but if I want to do a reaction at something like -0.10V the previous reaction would interfere with that happening(assuming
I am driving electrode potential negatively). |
Electrochemistry needs the details to be well defined or you may run into confusion.
Firstly, are these the half reactions you are referring to?
AgBr(s) + e- <=> Ag(s) + Br-(aq)...................E = +0.07133V
SnO(s) + 2 H+ + 2 e- <=> Sn(s) + H2O..........E = -0.10V
Now you say you're "driving the electrode potential negatively", I am not sure what you mean by this. Do you mean you are using it in this direction
as written above and your other electrode is an oxidation reaction with more positive electrode potential, e.g.
Fe(s) <=> Fe(2+)(aq) + 2e-.........................E = +0.44V
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
What I meant was applying an external voltage after the cell has reached it's open circuit potential. I think I understand these concepts now. Still
don't know exactly how to go about calculating the open circuit potential. Sorry for the delay, I've been completely swamped!
The cell values I gave were completely arbitrary. My current understanding of deciding whether electrodes will have side reactions is literally
looking up all the reactions I can think of in a table.
|
|
|
| Pages:
1
2 |