| Pages:
1
2 |
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Good Vacuum Distillation Experiment
Hi,
I am new to this forum, but I am not exactly new to chemistry. So anyway... I recently bought a bunch of new equipment. Seeing how lab equipment is
expensive, my equipment is pretty minimalist, and some of it is jerry-rigged, so I thought it would be a good idea to test it out before carrying out
any procedures with dangerous, expensive, or toxic reagents. Right now I am conducting a Distillation of Water experiment, which is proceeding
smoothly aside from the cloying scent of baby oil being emitted from the heating bath. (I should probably construct a hood....)
I'd like to test out my vacuum distillation setup, and I'm not really sure what to distill... vanilla flavoring perhaps... does anyone have any good
recommendations for OTC products to use for testing vacuum distillation equipment?
|
|
|
j_sum1
Administrator
       
Posts: 6225
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
DCM from paint stripper?
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I'm going to distill some paint stripper soon (that's why I am testing the equipment with water), but I was planning on doing that distillation at
atmospheric pressure....
|
|
|
RareEarth
Hazard to Self
 
Posts: 69
Registered: 1-4-2015
Member Is Offline
Mood: No Mood
|
|
DCM from paintstripper is a good suggestion. DCM is pretty valuable for extracting organics, and often in paintstripper it is mixed with methanol,
water, and other surfactants that make it impossible to use for those purposes.
However I'm not really sure you need a vacuum for distilling it. Pretty much anything from the hardware store would be a good choice for you.
|
|
|
j_sum1
Administrator
       
Posts: 6225
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by RareEarth  | DCM from paintstripper is a good suggestion. DCM is pretty valuable for extracting organics, and often in paintstripper it is mixed with methanol,
water, and other surfactants that make it impossible to use for those purposes.
However I'm not really sure you need a vacuum for distilling it. Pretty much anything from the hardware store would be a good choice for you.
|
You don't need a vacuum. But as its boiling point is so low, you can vac distill it without heating at all.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Vacuum distilling dichloromethane would be a nightmare, not even ice water would condense it if the pressure was low enough. To test out your vacuum
distillation apparatus, perhaps you could distill something reasonably innocuous, but with a high boiling point, such as glycerol. That should
provide a reasonable test and, if an incident occurred, you wouldn't have to worry about any toxic chemicals being present during cleanup.(Be sure
that you are equipped to handle the case of the glass imploding though!)
[Edited on 10-26-2015 by gdflp]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Whoah, my mineral oil bath started boiling profusely at atmospheric pressure at 115 C!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I was going to suggest anti-freeze (bp 197°C) (ethylene glycol) but glycerol is better as it is non-toxic. Then use the nomograph below to
determine your vacuum or check your results:
http://www.sigmaaldrich.com/chemistry/solvents/learning-cent...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I think Distillation of Glycerol is an excellent idea.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Using vacuum distillation, it is possible to break the ethanol-water azeotrope.
I haven't tried it as it requires very low temperatures.
But at high altitude, I have discovered the azeotrope is 96% rather than the
sea level 95.5% azeotrope.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I'm certainly not going to recommend Equate brand mineral oil to anyone.
|
|
|
j_sum1
Administrator
       
Posts: 6225
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by gdflp  | Vacuum distilling dichloromethane would be a nightmare, not even ice water would condense it if the pressure was low enough. To test out your vacuum
distillation apparatus, perhaps you could distill something reasonably innocuous, but with a high boiling point, such as glycerol. That should
provide a reasonable test and, if an incident occurred, you wouldn't have to worry about any toxic chemicals being present during cleanup.(Be sure
that you are equipped to handle the case of the glass imploding though!)
[Edited on 10-26-2015 by gdflp] |
Thanks for that. I was obviously misinformed.
I was merely passing on what I had heard and not what I had done.
|
|
|
zombiedude1
Harmless

Posts: 36
Registered: 15-10-2015
Member Is Offline
Mood: No Mood
|
|
Why not do a vacuum distillation of ethanol? 
Perhaps some wine.
Get yourself a proof and tralle hydrometer to measure the ABV content before and after the distillation with a vigreux column. Keep in mind, a vigreux
doesn't provide much reflux to dramatically increase ABV; it's essentially a "pot" still. The taller the column, the better. Combine two maybe? I'd
recommend a packed column but I'm not sure if it's a good idea with a vacuum.
(I personally haven't had the chance to perform a vacuum distillation yet outside of class, my water aspirator hasn't came in the mail yet.)
[Edited on 30-10-2015 by zombiedude1]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I already picked up some glycerol  I would prefer to do a vacuum distillation of
something that boils well above 100 C. Also, I'm not trying to have the ATF's jackbooted thugs break down my door and take away my lab over a little
glass of brandy I would prefer to do a vacuum distillation of
something that boils well above 100 C. Also, I'm not trying to have the ATF's jackbooted thugs break down my door and take away my lab over a little
glass of brandy 
[Edited on 30-10-2015 by JJay]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Are you sure you don't have moisture that's boiling out? I use dibutyl pthalate as my hot bath.
why is distilling DCM a "nightmare?" I've done it several times. It condenses above RT. A small amount of evaporation helps cool the receiver... to
almost "0"
First of all let me congratulate you on your practice distillations. I was having laboratory blues until I decided to stop synthesizing and practice
procedures. I did that for almost a year. You might enjoy distilling:
gasoline
fiber glass thinner
plumbers acid (h2so4)
make some azeotropes and prove them by distilling them
[Edited on 30-10-2015 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
He meant that it was a nightmare at lowered pressure as it would be hard to condense. If I remember correctly it boils at 40 C at 1atm, meaning it
would boil at an even lower temperature (and thus be harder to condense) at lower pressure.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
DCM is a real pain in the arse for noobs like me.
It just buggers off when you're not looking, same as Benzene.
I can see how it can be really useful in the right hands, however those would be Seriously well Experienced hands, not mine.
Vac + DCM = dichloromethane in the vac pump oil.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by chemrox  |
Are you sure you don't have moisture that's boiling out? I use dibutyl pthalate as my hot bath.
why is distilling DCM a "nightmare?" I've done it several times. It condenses above RT. A small amount of evaporation helps cool the receiver... to
almost "0"
First of all let me congratulate you on your practice distillations. I was having laboratory blues until I decided to stop synthesizing and practice
procedures. I did that for almost a year. You might enjoy distilling:
gasoline
fiber glass thinner
plumbers acid (h2so4)
make some azeotropes and prove them by distilling them
[Edited on 30-10-2015 by chemrox] |
I don't know what caused the mineral oil to boil, but it was boiling hard... I don't think that there could have been that much water in the mineral
oil. I started using a soybean oil bath instead, but I am thinking about switching to mineral oil, paraffin wax, or stearic acid... dibutyl phthalate
is an interesting idea also.
DCM will blow the stopper right off of a flask at room temperature... I'm definitely not trying to vacuum distill it; I'm going to use glycerine for
testing my vacuum distillation equipment.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
DCM in a bottle is OK so long as room temperatture is under 40 C.
Shit out of luck over here in high Summer.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Glycerine sure is hard to distill! I ran an unsuccessful distillation yesterday using a 500 watt immersion heater, and today, I used 1000 watts of
immersion heaters.
I got the glycerine boiling at around 130 C, but the vapor didn't seem to be making it out of the flask... I raised the soybean oil bath temperature
to 200 C.... a few drops of glycerine were forming in the bottom inch of my column.... I might get some product if I use a higher temperature bath and
remove the column and add some insulation.
Vacuum Distillation of Glycerine Trial 2 Temperature Log
A: Time
B: Bath Temperature (C)
C: Temperature at Condenser (C)
D: Notes
A B C D
1230 26 22 Power 100%
1233 54 24
1236 79 24
1238 96 25
1240 107 25
1242 126 26
1244 137 26 Some bubbles starting to form in flask - reducing heat to 50%
1247 146 27
1249 149 27 Gentle boiling started
1254 169 28 Power back at 100%
1256 177 28
0100 194 28
0102 200 Bath temperature is too hot - aborted
Mineral oil should be able to reach 300 C (I am using a 2 L bath). A lot of people use aluminum foil for insulation... that may be sufficient... I
think fiberglass could work well.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
What was your pressure (if you can measure it)?
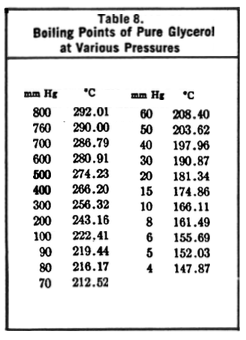
(If your glycerol has water in it, these values will change.)
[Edited on 1-11-2015 by Cheddite Cheese]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
My pump is rated at 6 microns. Temperature in the flask was likely about 20 C below temperature of the bath when the boiling started. I have a
pressure gauge, but it's a piece of garbage.
The glycerine is health food grade and claims to be "100% glycerine." It very likely contains some water, though.
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Tonight, I attempted to vacuum distill DCM from 1qt of paint stripper containing between 80-85% DCM. I used a pot of lukewarm water to heat the
boiling flask. I used a vacuum pump at 20 mmHg. A 200mm graham condenser was used with a 157 GPH pond pump and it was placed in bucket of ice water
along with the receiving flask; however, I observed the product in the collection flask boiling while on ice! The water bath was kept lukewarm during
the duration of the distillation, and ice was added as needed to the condenser pump bucket. Everything went well and rapidly for about 30 minutes,
then slowed to a halt. Only 150mL of product was collected. I then transferred the gel remaining in the boiling flask back to the original
container: it barely filled a quarter of the container. I also noticed a liquid in the tubing going to the vacuum, presumably DCM. I've done this
before with different brand paint stripper containing 60-65% DCM and have always had an awesome yield. Any idea why it didn't go too well?
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Why on earth would you put a vacuum on DCM? A pot of warm water is quite sufficient to distill it at atmospheric pressure and a high surface area
condenser fed with ice water is needed for efficient condensation. By applying a vacuum you probably just made it impossible to properly condense and
sucked it out as gas through the pump.
The point of vacuum distillation is to lower the boiling point of compounds sufficiently high boiling that they would decompose or cause undue thermal
stress on equipment during distillation.
A sane experiment to learn how to use vac distillation would be to prepare ethylene glycol from antifreeze concentrate. Normally boiling at 197C,
aspirator vacuum will cause it to pass over at 95-100Cish.
[Edited on 7-11-2015 by UC235]
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
I am an impatient noob. Sometimes you gotta learn the hard way... but I didn't really because I wasn't injured. We may have to wait 20yrs. before I
find out.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
| Pages:
1
2 |