Quatro
Harmless

Posts: 22
Registered: 31-10-2015
Location: Arizona
Member Is Offline
Mood: Nucleophillic
|
|
Discrepancies in Shulgin's Synthesis? Tryptamine + MeI = 1-Me-TMT?
Alkylating tryptamine with a methyl halide will produce a tetraalkylammonium product.
Shulgin reacts methyl iodide and tryptamine to form N,N,N-trimethyltryptammonium iodide. This is the PREFERRED product. He yields 60% of this
"TMT". He also claims that the side products will be DMT, NMT, and Tryptamine. I looked up any information I could about this quaternary amine salt,
and stumbled upon this article.
| Quote: |
All yields are summarised in Table 1. The data supports the formation of 47.4% of 1-Me-TMT and 21.0% TMT from the Breath of Hope synthesis involving
the reaction of tryptamine with methyl iodide. |
The article is a bit confusing at some parts, and I can't tell if they employed different methods than Shulgin did. Why did they only yield 21% TMT,
when Shulgin yielded 60%?
From some research, I learned that:
| Quote: |
The 1 position of indole is difficult to alkylate. Very strong bases are usually needed. |
| Quote: |
The scenario of the indole amine getting methylated isn't likely (probably due to the pi-bond character in pyrrole...that amine is less amenable to
protonation/deprotonation in the conditions being considered here) but formation of the quat-amine (N,N,N-TMT) is. |
What am I missing? I thought the indole amine was hard to methylate? Theoretically, I would like to yield as much TMT as possible. (Maybe let the
reaction run longer than Shulgin did?)
Also, the previous reference claims that only a MINUSCULE amount of NMT is produced, and there was NO DMT side product whatsoever. (Shulgin claimed
that enough DMT side product is produced that it could be purified into the picrate salt)
I attached pictures of 1-Me-TMT and TMT if you are interested.
Thanks for helping me understand guys! 
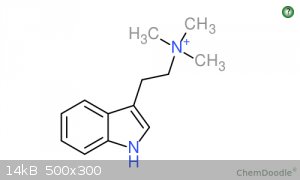
[Edited on 1-11-2015 by Quatro]
[Edited on 1-11-2015 by Quatro]
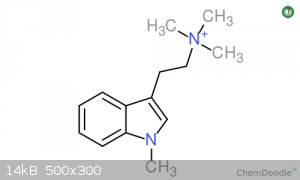
[Edited on 1-11-2015 by Quatro]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
The paper by Martins et al had a few different parts worth differentiating. The focus of the paper, and the part you are also referring to, is about
the "breath of hope" synthesis. This method uses sodium hydroxide and phase transfer catalysis. Martins et al also used Shulgin's method (modified)
to make N,N,N-trimethyltryptamine iodide, where they got a 48% yield.
The methylation of the indole nitrogen is a bit surprising. That result may have had something to with their use of phase transfer catalyst along
with the sodium hydroxide. I doubt that the ring nitrogen gets methylated under the neutral conditions Shulgin uses to get the quaternary salt.
In their modification of Shulgin's method, I noticed that Martins et al only ran the reaction for 2 hours, where Shulgin allowed it to run for 12
hours. That probably explains his higher yield. Perhaps that minuscule amount of N-methyltryptamine left at the two hour point is converted to a
minuscule amount of dimethyltryptamine when the reaction is allowed to proceed for another ten hours. However, Shulgin himself wrote that the yield
of DMT from this is minuscule, and says that it should be present at all only in principle.
[Edited on 1-11-2015 by Crowfjord]
|
|
|
Quatro
Harmless

Posts: 22
Registered: 31-10-2015
Location: Arizona
Member Is Offline
Mood: Nucleophillic
|
|
Ahhh thank you very much for the explanation! I should have noticed their use of NaOH...
If you were to run the reaction under the neutral conditions Shulgin uses, would there be a way to optimize the reaction, and yield higher amounts of
the quat salt? (Shulgin gets about 60%)
10g of methyl iodide is more than 3 molar equivalents for the Tryptamine used. It seems only small amounts of NMT are produced, (minuscule amounts of
DMT), and mostly unreacted Tryptamine.
Theoretically, how could TMT yield be improved? Running the reaction even longer?
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
I think that in the synthesis of quaternary amines from primary amines, a sterically hindered base must be used. Something like 1,2,2,5,5-pentamethyl
piperidine or diisopropylethylamine, that will scavenge formed HI without itself getting methylated. Without the base, it's going to be tryptamine,
N-methyltryptamine, and dimethyltryptamine that neutralize the formed acid. The positively-charged amine hydroiodides won't be nucleophilic enough to
react with further methyl iodide. Of course, base seems to increase the chance of 1-methylation. Perhaps in this case, 60% yield is about as good as
it gets.
|
|
|
Quatro
Harmless

Posts: 22
Registered: 31-10-2015
Location: Arizona
Member Is Offline
Mood: Nucleophillic
|
|
Very elegantly put Crowfjord! That makes sense... A sterically hindered base might not be advantageous due to higher risk of the 1-indole
alkylation...
I think you're right, 60% will probably have to do.
|
|
|
Quatro
Harmless

Posts: 22
Registered: 31-10-2015
Location: Arizona
Member Is Offline
Mood: Nucleophillic
|
|
After filtering the solids from the solution, Shulgin gets a mixture of Tryptamine, NMT, DMT, and TMT. He then washes this twice with IPA to isolate
pure TMT.
I know Tryptamine, NMT, and DMT are all soluble in IPA. And they are all very similar in structure. Why is it that adding a methiodide group to DMT
suddenly makes it completely insoluble in IPA?
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
I think using PTC in two phase alkylation is a very bad choice to alkylate the N ring of tryptamines because before the deprotonation of the NH the
iodomethane and the tryptamine will stay in the organic phase and react together to form quaternary anion.
IMo the best option is to use a monophasic system. Dissolving the tryptamine into an aprotic polar solvent such as DMSO or DMF, then deprotonating the
NH with a strong base such as NaH then adding the MeI or other alkylating reagent.
|
|
|
Quatro
Harmless

Posts: 22
Registered: 31-10-2015
Location: Arizona
Member Is Offline
Mood: Nucleophillic
|
|
DrMethyl, I'm not sure if I'm following you. The goal is to avoid methylation of the indole nitrogen.
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
ohhh sorry,
the nitrogen of the indole wont get methylated because the Nitrogen is not nucleophilic because its electron pair is take part of the aromaticity of
the indole.
|
|
|
Syntropymancer
Harmless

Posts: 5
Registered: 14-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Quatro  | After filtering the solids from the solution, Shulgin gets a mixture of Tryptamine, NMT, DMT, and TMT. He then washes this twice with IPA to isolate
pure TMT.
I know Tryptamine, NMT, and DMT are all soluble in IPA. And they are all very similar in structure. Why is it that adding a methiodide group to DMT
suddenly makes it completely insoluble in IPA? |
Quaternary salts have a permanent formal charge, drastically changing the solubilty. Think of it like any other positively charged salt, except, it
doesn't dissociate, it decomposes.
[Edited on 15-12-2015 by Syntropymancer]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Quatro  |
From some research, I learned that:
| Quote: |
The 1 position of indole is difficult to alkylate. Very strong bases are usually needed. |
|
Quote: Originally posted by DrMethyl  |
the nitrogen of the indole wont get methylated because the Nitrogen is not nucleophilic because its electron pair is take part of the aromaticity of
the indole. |
I am quite surprised by your posts.IMHO, the indole N is not that difficult to alkylate.In fact it is quite reactive.That's why in most indole
reactions,you have the protect the indole N.
Quote: Originally posted by Quatro  | A sterically hindered base might not be advantageous due to higher risk of the 1-indole alkylation...
|
How ?
won't the sterically hindered base have dificulty deprotonating the indole N rather than the primary N and thus decrease indole N alkylation ?
Actually,the best option is to not go by this route at all. The alkylation of the indole N is a common problem which is difficult to prevent.That's
why most of the routes use the dialkylated amine directly instead of putting the amine first and then alkylating it.
[Edited on 15-12-2015 by CuReUS]
|
|
|
dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
I've always wondered why he would go to such lengths to overshoot then demethylate to achieve such a bad yield. 0.12g from 3.00g is horrible for a 3
product system.
Wouldn't it be more efficient to monitor the reaction at the 8hour mark via TLC and run a column for the DMT, TMT, and nMT? That would be easier,
quicker, and yield more results than 120mg out of 3g of tryptamine, no?
|
|
|