| Pages:
1
2 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
2-octanol
Introduction
7.6g of 2-octanol was prepared by pyrolysis of castor oil using NaOH. The synthesis was a 1/6 scale version of the method of Vasishtha et al (ref 1)
combined with a modified equipment arrangement described in the OrgSyn procedure for methyl-n-hexylcarbinol (ref 2). 22.9g of sebacic acid was
recovered as a by-product. This is reported in a separate thread (ref 3).
Reagents & Equipment Set-Up
The OrgSyn procedure highly recommended the use of a copper pyrolysis vessel. In copper I was limited to a 500ml RBF. Due to the necessarily small
scale of my procedure I had to prepare 5 batches.
25g of castor oil (Rite-Aid) was first saponified with 3.3g of NaOH in 75ml of water. To this was added 0.25g of red lead, 66.7g of Up & Up
(Target) “heavy duty” mineral oil, and an additional 16.7g of NaOH. This was placed in the copper RBF. A magnetic stir bar was added and the RBF
placed on an electric heating mantle. The RBF was equipped with a Claisen adapter to allow pot temperature measurement by thermocouple and a
condenser assembly. The condenser assembly consisted of a vertical West condenser, a U-tube, and a vertical Graham condenser as shown below. The
West condenser was equipped for steam heating; the Graham condenser was cooled with ice-water.
Procedure
The pot was insulated with a fiberglass blanket and heated. The West condenser was left unheated until ~55ml of water condensate had collected in a
graduated cylinder receiver. At this point a trace of 2-octanol could be detected in the condensate. Pot temperature at this point was about 180°C.
The receiver was then changed to a 125ml separatory funnel and steam heat was applied to the jacket of the West condenser. Heating was continued
until no more 2-octanol came over. The final temperature in the pot had risen to about 325°C.

Preliminary water removal

Pyrolysis collecting 2-octanol
Workup
The pot residue contained a sebacic acid by-product. The workup of this is described in reference 3. The combined distillate of crude 2-octanol was
40ml for the 5 batches and is shown in the picture below drying over K2CO3.

crude 2-octanol
The crude 2-octanol was placed in a 100ml RBF for fractional distillation using a Hempel column packed with glass shards (bp 178.5°C per Wiki). This
column had to be abandoned due to flooding.

Hempel column flooding
A Vigreux column was then tried. This worked well until about half way through the distillation. Then the column flooded due to severe foaming. I
tried many things to get rid of the foam: (1) added magnetic stirring, (2) removed boiling stones, (3) tar removal using activated carbon, (4) added
a few drops of baby Simethecone (Safeway), a polydimethylsiloxane, and (5) add a few drops of DOT5 silicone brake fluid (a diorganopolysiloxane).
Nothing worked except the DOT5 silicone oil. It stabilized the foam but did not prevent its formation. The presence of a layer of foam (~7mm) was
enough to prevent evaporation of the 2-octanol and hence prevent distillation. The results were: (1)10ml light forerun (bp<170°C), (2) 20ml of
middle cut (bp 170-176°C), and (3) 8ml of dark amber pot residue.
The middle cut was placed in a 50ml RBF and redistilled. There was a forerun of about 5ml that boiled at 170-175°C, probably mostly 2-octanone (bp
=172.9°C). The remainder of the distillate (6.6g) came over at 175-176°C and was taken as 2-octanol per OrgSyn. There was only a slight bit of tar
as pot residue. There was no foaming during the distillation.
The forerun was combined with the forerun of the first distillation. This 13ml was distilled using a 25ml pot. 1 additional gram of 2-octanol was
recovered boiling at 175-176°C. Again there was no foaming and only a slight bit of tar.
Results
The yield of 2-octanol was 7.6g for a %yield of 23.8%.
Discussion
The %yield of this synthesis is disappointing. Vasishtha (ref 1) claims a yield of 70.1%. OrgSyn (ref 2) claims yields of 40-42%. Discussed below
are some factors that may have contributed to my low yield.
a. Tar & Foaming
Much time was spent trying to distill the crude distillate because of foaming. To get the distillate to rise to the top of a 20cm fractionation
column required a lot of heat: 80% setting on the mantle and heavy insulation of the pot and column. This degraded the distillate as I could see it
becoming progressively darker. If a vacuum distillation could be used this should greatly reduce tar formation. I found it ironic that in my search
for an antifoam I learned that 2-octanol itself is an antifoam for some applications. Indeed, in the absence of the tar there was no foaming problem.
When cleaning the glassware I found it difficult to form suds with liquid detergent, confirming that 2-octanol is an antifoam.
Trying the baby Simethicone was ill-advised. Although it is a polydimethylsiloxane it is in an aqueous dispersion and therefore was not compatible
with the fatty 2-octanol.
b. Equipment Configuration
Both the Vasishtha and OrgSyn procedures specified a pyroysis equipment configuration in which condensate water would be returned to the pot. I did
mimic the OrgSyn configuration, in a modified way, but did not provide for condensate return to the pot. It was never clear to me why this water
return was necessary. Perhaps this was a way to extend the time of pyrolysis. Vasishtha specified a time of 5 hours, and OrgSyn specified a time of
48 to 72 hours! However, my yield of sebacic acid was 84.3% for the last two batches whereas that for Vasishtha was 72.5%. Based on this I conclude
that my pyrolyses were complete even though my average pyrolysis time was only 2.3 hours.
References
1.”Sebacic Acid and 2-Octanol from Castor Oil,” by A.K. Vasishtha et al, Journal of American Oil Chemists, vol 67, no. 5, May 1990. http://www.sciencemadness.org/talk/viewthread.php?tid=62326&...
2. “Methyl-n-hexylcarbinol (2-octanol),” by Roger Adams et al, Organic Syntheses, Coll. Vol. 1, p.366 (1941). http://www.orgsyn.org/Result.aspx
3. http://www.sciencemadness.org/talk/viewthread.php?tid=64038#...
Comments, questions, and suggestions are welcomed.
[Edited on 4-11-2015 by Magpie]
[Edited on 4-11-2015 by Magpie]
[Edited on 4-11-2015 by Magpie]
[Edited on 4-11-2015 by Magpie]
[Edited on 4-11-2015 by Magpie]
[Edited on 4-11-2015 by Magpie]
[Edited on 4-11-2015 by Magpie]
[Edited on 4-11-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2693
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | It was never clear to me why this water return was necessary. Perhaps this was a way to extend the time of pyrolysis. |
Pyrolysis is weird. Water could have participated in the reaction; one possible mechanism assumes that C12-OH is oxidized and undergoes an ene
reaction with C9=C10 so that C9 bonds to oxygen, then that ether must be cleaved, which probably involves water somehow.
I wouldn't change a procedure unless I thought I knew what it was doing. Furfural from corn cobs or some shit, do it by the book.
[Edited on 4-11-2015 by clearly_not_atara]
[Edited on 4-11-2015 by clearly_not_atara]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Perhaps the water was intended to provide steam distillation of the 2-octanol.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
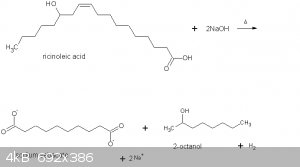
Magpie,
What's the mechanism of the reaction that you show in your graphic? It's quite a strange/interesting redox reaction as the double bond is cleaved to
give you the two products. Can't say that I have ever seen anything like this (though I have not looked either).
And, as usual, an excellent presentation.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you, AvB. A proposed mechanism is shown on the 3rd page of the Vasishtha reference. The link in reference 1 will take you to p. 9 in
References. Scroll down over half way and you will see Solo's link to the article. In summary:
1. dehydrogenation of ricinoleic acid
2. isomerization of the ß, gamma - keto acid
3. retro-aldol fission to 2-octanol & sebacic acid
I'm sure you will understand this much better than I do.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 126
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
Hey Magpie,
this is a good patway to get this alcohol. I like it. Do you have some similar methode to get pentanol?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Not offhand. I think the double bond and the hydroxyl group in the ricinoleic acid makes this synthesis possible. I have also wondered if other fatty
acids might have these functional groups.
As you know fatty acids are easily attainable through saponification of agriculturally derived oils.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 126
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
Ok this is evidence, but i thought not this castor oil as starting material, I asked some similar pyrolysis methode from other plant oil.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I redistilled the forerun and obtained 5.9g of liquid boiling at 172-174°C. Assuming this to be mostly 2-octanone (bp 172.9°C) I reacted a portion
of it with semicarbazide to form 2-octanone semicarbazone. This, shown below, had a mp =123°C in agreement with the literature value (Brewster).

[Edited on 7-11-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Magpie  | Thank you, AvB. A proposed mechanism is shown on the 3rd page of the Vasishtha reference. The link in reference 1 will take you to p. 9 in
References. Scroll down over half way and you will see Solo's link to the article. In summary:
1. dehydrogenation of ricinoleic acid
2. isomerization of the ß, gamma - keto acid
3. retro-aldol fission to 2-octanol & sebacic acid
I'm sure you will understand this much better than I do. |
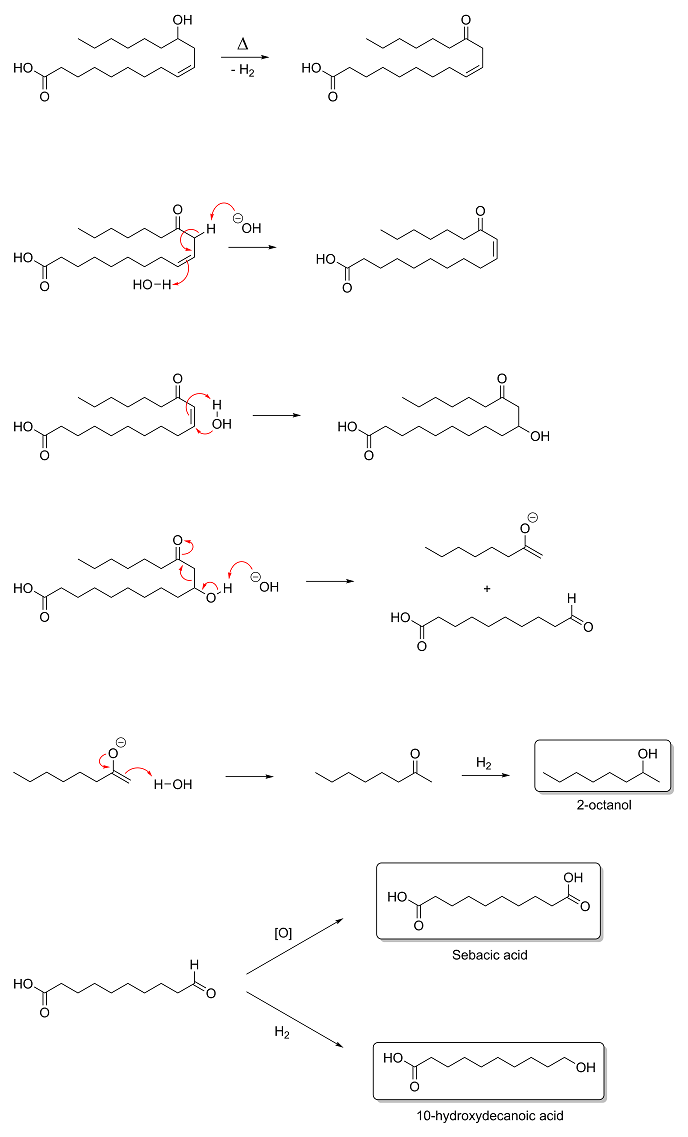
The retro-aldol fission step gives methyl hexyl ketone and 10-oxodecanoic acid, not 2-octanol and sebacic acid. It's the subsequent reduction of
methyl hexyl ketone by the H2 produced in the first step that gives 2-octanol, and the oxidation of 10-oxodecanoic acid that gives sebacic
acid; however, the 10-oxodecanoic acid can also be reduced by H2 as well to give 10-hydroxydecanoic acid, which seems to be the point of
adding the Pb3O4 so that oxidation is favored. Based on the mechanism suggested in the Vasishtha reference, I imagine the
reaction looks something like this:
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
If this actually works, this is a great prep!I suppose there is no way to guarantee the isomer of 2-octanol that is produced [or does the melting
point mach only with the 2- isomer?].
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
2-octanol is a liquid at room temperature. You could look at BP (178.5°C relative to 1-octanol at 195°C ), though. Many of the other isomers,
however, have similar BPs, so GC would probably be the only way to go. I'd run this on a wax column.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Interesting. Though the fact that it wa not n-octanol was determined by the oxidation since it obviously made a ketone, not an aldehyde.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Darkstar,
Thanks for the explanation. I completely missed the addition of red lead in the reaction in Magpie's write up. Knowing that red lead is present helps
clarify the seemingly strange redox reactions going on. Nice post!
AvB
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Splicing the nonoleic acid using potassium permanganate and aliquat 336 would give interesting compounds (albeit, rather profitless ones). I think
they would be separable with distillation, though it'd be close. How feasible would that be?
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by AvBaeyer  | Darkstar,
Thanks for the explanation. I completely missed the addition of red lead in the reaction in Magpie's write up. Knowing that red lead is present helps
clarify the seemingly strange redox reactions going on. Nice post!
AvB |
Yeah, the reaction Magpie originally depicted in the image threw me for a loop too. There should have been a water on the reactant side and no
H2 on the product side. After dehydrogenation and isomerization of ricinoleic acid, water adds to the α,β-unsaturated ketone to give an
aldol that undergoes retro-aldol fission to give the corresponding enolate and aldehdye. The enolate is then protonated to give a ketone (2-octanone)
which gets reduced to 2-octanol by the H2 generated in the dehydrogenation step. The aldehyde (10-oxodecanoic acid) is then either oxidized
to sebacic acid or reduced by H2 to 10-hydroxydecanoic acid. It is certainly an interesting reaction.
I don't blame Magpie, though. The mechanism in the reference is sort of hard to follow. For those who don't have access to the References section,
here's the mechanism proposed in the Vasishtha reference (and what my mechanism above is based on):
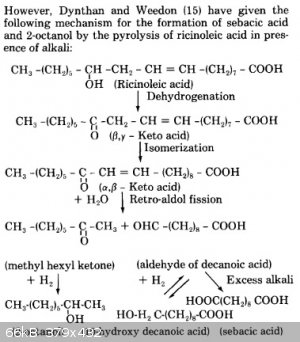 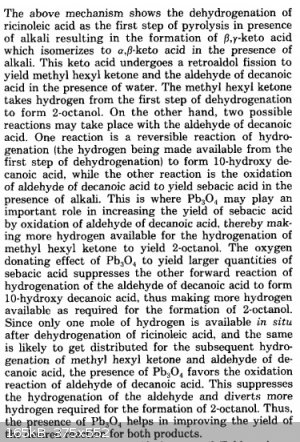
The ketone in the forerun was unreacted 2-octanone (methyl hexyl ketone), not 2-octanol that got oxidized back to a ketone at some point. Like I said
above, it's 2-octanone that results from the retro-aldol fission step that must then get reduced by H2 to the secondary alcohol. I think
the reason Magpie got such high yields of sebacic acid but such low yields of 2-octanol was due to incomplete reduction. Maybe it had something to do
with him altering the procedure (water return)? No protons for the enolate ions to grab and thus no ketones to be reduced to 2-octanol?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
That is an interesting mechanism, I think it would have gotten a low grade in my advanced organic class, but it is hard to argue against empirical
evidence that something skin that that happens, given the final products. Perhaps the lead acts as some sort of redox cycler in the shuttle of the
hydrogen back and forth, it may even be a sort of transfer hydrogenation. It might be that conducting the reaction in a more sealed container would
help keep the hydrogen so that it can be recycled, or even adding another reductant than can generate hydrogen, such as ammonium formate, hydrazine,
or a THP compound, or another of the classic transfer H sources. Also wonder if Pd would catalyze the reaction as well or better. But it clearly
works to some degree. The use of a copper vessel also might be a way to get traces of Pt/Pd/Ag into the reaction, as most copper has traces of it,
and at higher temps, traces of those might be dissolves. Just look at "Pd free cross couplings" to find similar examples of very small traces of
transition metals having a huge effect.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Darkstar  |
Yeah, the reaction Magpie originally depicted in the image threw me for a loop too. There should have been a water on the reactant side and no
H2 on the product side. .... |
Here's a quote from Organic Syntheses procedure for methyl-n-hexylcarbinol (2-octanol):
| Quote: |
The container is now fitted with an efficient reflux condenser, and it is then heated over a ring burner as long as hydrogen is evolved. The heating
is regulated to produce a fairly rapid evolution of gas as shown by leading a tube from the top of the condenser into a small beaker of water. The
time required for the complete evolution of the hydrogen is about nine to ten hours. |
I do remember some gas bubbling (evolution) during the synthesis. I must have had the outlet of the Graham condenser submerged in the receiver.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I just mean for a balanced reaction is all. One ricinoleic acid molecule gives one H2 molecule and then reacts with two hydroxide ions and
one water molecule to give one 2-octanone molecule and one 10-oxodecanoic acid molecule. The 2-octanone then reacts with the H2 to give
2-octanol, and the 10-oxodecanoic acid gets oxidized to sebacic acid. So something like this:
ricinoleic acid + 2 NaOH + H2O + heat → 2-octanol + sebacic acid
The H2 is both formed as well as consumed during the reaction itself, so it's technically neither a starting reactant nor a final product.
In practice hydrogen will certainly evolve since not all of it is going to react with 2-octanone or 10-oxodecanoic acid. Again, I just meant for a
balanced equation. It's definitely an interesting reaction to say the least, and I must say that this was a wonderful write-up. I just wanted to help
clarify what the Vasishtha reference suggests since there was clearly some interest in this rather strange reaction mechanism.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
But my reaction is balanced and the product is sodium sebacate, not sebacic acid. Only after the addition of HCl does one aquire sebacic acid.
I made an error in portraying the ricinoleic acid as some readers may have noticed. The -OH and the double bond need to be moved one carbon to the
right.
[Edited on 12-11-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
If you noticed, I never bothered to show sebacic acid as sodium sebacate in my mechanism either. (and neither did the Vasishtha reference) Yes, it
will be deprotonated under such basic conditions; however, my main point was to show the part of the reaction that we are actually concerned with. I
wanted to show two things: that water does participate in the reaction according to the Vasishtha reference, and that H2 is
generated in situ and then consumed and not a product. How you want to treat the NaOH and water on either side is up to you. If you look at
the mechanism, hydroxide actually gets regenerated in the pyrolysis part of the reaction and isn't technically consumed.
By balanced I really just mean that you don't have one mole of ricinoleic acid somehow generating two moles of H2 like in the original
image. Because if H2 were to evolve like in your original image, there wouldn't have been any 2-octanol--it would've been 2-octanone. This
started when you said:
| Quote: | | 3. retro-aldol fission to 2-octanol & sebacic acid |
I initially just wanted to point out that there is one last step that you missed, which is the reduction of 2-octanone (which is what the retro-aldol
fission gives) to 2-octanol by H2, which is probably the most interesting part of this reaction to begin with. I hope I have not caused too
much confusion.
[Edited on 11-12-2015 by Darkstar]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for your insights and interest, Darkstar.
I have some disappointing, although not totally surprising, lab results to report. It seems that my putative 2-octanol is 2-octanone. Looking back,
my first clue was the low boiling point of 175-176°C. The bp for 2-octanol is 178.5°C.
Yesterday I attempted to make 2-octyl hydrogen phthalate. This failed. Today I tested my putative 2-octanol with (1) Na and (2) with acetyl
chloride. The Na did produce a bubbling (H2) but not profusely. Acetyl chloride produced no reaction. Then I reacted some with semicarbazide and
produced a nice mass of crystals looking just like the 2-octanone semicarbazone that I made before.
So, I will try this synthesis again. I will be trying to avoid conditions such as overheating that would promote oxidation of the 2-octanol.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Do you have any of the 2-octanone left? If so, you might be able to recover some 2-octanol by reduction with aluminum isopropoxide or sodium
borohydride.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I don't have those reagents, but thanks. I want to get the castor oil procedure to work. I've invested a lot of time and effort in it.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
The fact that you isolated 2-octanone is interesting in its own right. Be that as it may, thanks again for the great write up and the instigation of
some interesting discussion. Even old timers like me can still learn new things.
AvB
|
|
|
| Pages:
1
2 |