| Pages:
1
2 |
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Copper(II) sulfate crystallisation in presence of ethanol
A solution of 20g of copper(II) sulfate pentahydrate in 75cm3 water at 95ºC was prepared in a beaker. Once it had all dissolved, 99%
ethanol was added dropwise with a Pasteur pipette, occasionally shaking slightly the beaker, until a very slight cloudiness was observed. The beaker
was left to cool at room temperature for the copper(II) sulfate to crystallise.
Observations
The copper(II) sulfate grew forming somewhat small blue needles similar in shape (but not identical) to those seen in benzoic acid, instead of
following the typical patterns.
Unfortunately, I did not record data for the twinning observed, but I strongly encourage fellow home chemists to attempt this and/or explain the
nature of this different crystallisation.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
What is the 'usual' crystal shape of copper sulphate ?
Rhombic ?
Perhaps the battle (for the water) between the Ethanol versus the copper sulphate pentahydrate affects the crystal shape.
Were the needles mostly pointing Upwards towards the Ethanol ?
[Edited on 13-11-2015 by aga]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
The ethanol was not visible as a separate layer and had most certainly mixed with the water. The crystals did not grow in any main direction.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Any photos ?
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
No. Sorry.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If there was enough ethanol, or the solution was warm enough, you might have formed the trihydrate rather than the pentahydrate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Why, DraconicAcid? (genuinely curious)
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Just had a go at this one.
Two 100ml beakers had 20g of copper sulphate pentahydrate mixed with 30ml of water, stirred, heated and dissolved.
To one beaker 100% ethanol was added dropwise and gently shaken into solution until a cloudiness was observed (3ml).
Both were left to cool to RT.
Crystal formation was quite rapid, with a thick layer of crystals after around 20 minutes.
The sample with the EtOH added does indeed appear to have a more 'spiky' appearance.
Here's a small sample of straight copper sulphate crystals under the microscope :

Now the ethanol treated sample
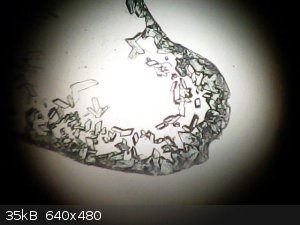
This is simply nice and blue

[Edited on 15-11-2015 by aga]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Copper(II) sulphate does form a stable trihydrate at slightly-higher-than-room-temperature (it was one of my compounds for the copper carnival). If
you're recrystallizing it from a solution that is not 100% water, then it's less likely to form the pentahydrate.
Think of the equilibrium CuSO4*5H2O = CuSO4*3 H2O + 2 H2O
If you decrease the concentration of water by adding ethanol (and if it's 10%, 20% ethanol, then water no longer counts as the solvent, and must be
included in the equilibrium expression), you shift the equilibrium to the left.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Having a look at the two pots a few hours after nightfall (temperature had dropped to 14 C) i have to retract my earlier comment about the
'spikyness'.
The only major difference is that the Ethanol treated crystals were less deep blue than the untreated crystals.
In physical appearance, they are the same - rhombic.
What exactly are you stating as 'different' in the shape over non-ethanol formed crystals ?
Your process will be re-tried using your precise quantities rather than those that i calculated.
Over what period of time did you allow the crystals to form, and what is the ambient temperature where your experiment was performed ?
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
I left them growing for about 6 hours. Ambient temperature around 18ºC.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Regarding your last post, aga, I have thought of a possible explanation. After reading through some of my notes of crystal growing with coordinated
water molecules, I've found out that for potassium bis(oxalato)cuprate(II), if it is left in quite a saturated solution, it will form a light blue
dihydrate. However, for more diluted solutions, compounds with more coordinated waters will form - yielding a dark blue thin needle crystal.
A very important observation for this though: if the initial saturated solution is left to crystallise for enough time, the concentration in solution
will change due to the rate at which water and the potassium bis(oxalato)cuprate(II) were crystallising... giving rise to the dark blue needles!!!
Hence, extrapolating, I think that this may explain why initially you might have been able to observe the thin needles for the copper(II) sulfate and
after enough time it started to crystallise normally. After having read about this, I'm inclined to think that a similar thing has happened to your
crystals.
As always, thank you very much for having put the effort to think about this, I really appreciate it 
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ah.
Makes sense.
I calculated the qty to use to saturate the hot solution hoping that it would accelerate the crystal formation.
I'll try your quantities when i get the current drop of crystals dry (so i can weigh them accurately) and post photos.
Any idea what volume of EtOH you used ?
Edit:
Why has nobody else tested Eddygp's observations yet ?
Is there a global shortage of copper sulphate, or just a shortage of any amateur scientists interested in anything other than random pointless Babble
?
For even the nooest noob, this one is easily do-able.
If the ethanol is a problem, simply producing a photo of un-treated home-made copper sulphate crystals would be helpful as a reference to what they
Should look like.
Perhaps the crystals would form differently if Other substances were added, e.g. cooking oil, Milk, gasoline ...
As a group, the SM Majority seems to be pretty Idle in terms of actually Doing any Chemistry, favouring the Banal instead.
(i'm also guilty in this).
[Edited on 18-11-2015 by aga]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I was planning to try something like this after I finish marking these lab reports.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Your efforts and results will be greatly appreciated.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Any results, DraconicAcid et al.?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not had a great deal of spare (sober) time to devote to it to be honest.
Certainly is on the list of things to do.
Hopefully this weekend.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I finally got around to doing some puttering.
I tried recrystallizing copper(II) sulphate from 5 M sulphuric acid, reasoning that the lower water concentration might favour a trihydrate. I got
some narrow, pale blue crystals, that don't really look like the pentahydrate (those lovely rhombic ones), so it's possibel that it worked. I haven't
had a chance to analyzing them yet, though.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
(seriously) As soon as the next *hic* batch of ethanol is ready think I might just have to try this.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I thought the Cu II sulfate pentahydrate was monoclinic but could be triclinic
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
prelim:
sat solution of copper sulphate pentahydrate split between two test tubes. 85%EtOH added to one and shaken, crystals salted out. When the EtOH I
drank wears off will repeat and MEASURE everything.

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The crystals I got from 5 M sulphuric acid were washed with methanol and dried at 50 C. They turned lighter blue, so some decomposition occurred, and
an oily residue was left on the plate. Either they were insufficiently washed, or crystallized with some sulphuric acid rather than just water. So
not the trihydrate like I had hoped.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | | The crystals I got from 5 M sulphuric acid were washed with methanol and dried at 50 C. They turned lighter blue, so some decomposition occurred, and
an oily residue was left on the plate. Either they were insufficiently washed, or crystallized with some sulphuric acid rather than just water. So
not the trihydrate like I had hoped. |
Going by most phase diagrams I've seen the lower hydrates seem to crystallise always at higher temperatures.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by DraconicAcid  | | The crystals I got from 5 M sulphuric acid were washed with methanol and dried at 50 C. They turned lighter blue, so some decomposition occurred, and
an oily residue was left on the plate. Either they were insufficiently washed, or crystallized with some sulphuric acid rather than just water. So
not the trihydrate like I had hoped. |
Going by most phase diagrams I've seen the lower hydrates seem to crystallise always at higher temperatures. |
From aqueous solution, that's to be expected. From a partially aqueous solution, with a lower concentration of water, that's not necessarily true.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
This is what I got the last time crystallizing copper sulphate solution (the solution was supersaturated and cooled rapidly in an ice bath). Is it
what you mean?

|
|
|
| Pages:
1
2 |