ItaBier
Harmless

Posts: 4
Registered: 17-11-2015
Member Is Offline
Mood: No Mood
|
|
phosphoethanolamine
Hi anyone have any information on the simple synthesis of phosphoethanolamine, because here in Brazil is much talked about for its anticancer
properties.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
It should be pretty easy to make. It's just the phosphoric acid mono-ester of monoethanolamine.
I'd say reflux monoethanolamine with a large excess of phosphoric acid for a couple hours, basify with sodium carbonate, then see what can be
distilled off under vacuum. Or, extract it with some inorganic solvent.
Not sure though. Sigma calls for -20C as a storage temp. Could be unstable.
[Edited on 17-11-2015 by Praxichys]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Amazingly, under the tuition of the B&D Science School, i finally have a clue what all of those words mean.
It's a Revelation !
|
|
|
Nicodem
|
Thread Moved
17-11-2015 at 12:42 |
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Boiling with phosphoric acid will not give any product. You need a specialized phosphorylating agent such as, for example, dibenzyphopshoryl chloride.
There is a huge literature on this subject (phosphorylation reactions and reagents) and it is relatively easy to find and learn from.
AvB
|
|
|
ItaBier
Harmless

Posts: 4
Registered: 17-11-2015
Member Is Offline
Mood: No Mood
|
|
Hi, i find the simple way, i see various syntheses with complex molecules, gold chloride, barium, etc. Already the largest university researchers have
invented a process by the reaction of the two compounds(mea and phosphoric acid) in an alcoholic medium, washinhg with ethanol and heating at 190
degrees to transform phosphate in phospho and neutralization with calcium and magnesium carbonates with yeld of 90%. the problem is the details, here
patents has no free access.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Well, phooey. For some reason I assumed phosphates were like borates. It does seem pretty straightforward with the right reagents though. All the
preps in Brauer use POCl3 so they're kind of prohibitive for amateur use.
This paper has an interesting route:
http://www.sciencedirect.com/science/article/pii/S0040403908...
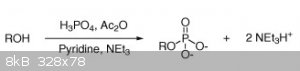
The only difficult thing to get here is the pyridine.
|
|
|
ItaBier
Harmless

Posts: 4
Registered: 17-11-2015
Member Is Offline
Mood: No Mood
|
|
Does there exist error in the following way: 1 mol ethanolamine + 1.1 mol of phosphoric acid + 1 mol of absolute ethanol at room temperature. wash the
precipitate with absolute ethanol. Heat the precipitate by 190 C for one hour. neutralizing with calcium carbonate and dissolve in hot distilled
water. wait for the crystallization and drying the precipitate (melting 240 C)
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I don't know about the difficulty in forming phosphate esters, but it's going to be a lot tougher to make an ester from ethanolamine than from
ethanol. The amine group is basic, and a better nucleophile than the hydroxyl group of the alcohol.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
It's not impossible that method works, but you can't check it with taught chemistry. The first step would produce a salt which might dehydrate to
produce the target on heating. The best way to check would be to look it up in proper literature.
There are a lot of desperate people wanting to believe in cures, something that can be cooked up from industrial chemicals sounds very risky to me.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Quote: Originally posted by ItaBier  | | Hi anyone have any information on the simple synthesis of phosphoethanolamine, because here in Brazil is much talked about for its anticancer
properties. |
Actually, here the phophoethanolamine made by Prof. Dr. Gilberto Orivaldo Chierice and his team was in the form of the mixed salts of Zn, Ca and Mg
using a modified synthesis (Ca-EAP, Mg-EAP, sold OTC in USA and Europe as supplements).
Quote: Originally posted by Praxichys  | It should be pretty easy to make. It's just the phosphoric acid mono-ester of monoethanolamine.
I'd say reflux monoethanolamine with a large excess of phosphoric acid for a couple hours, basify with sodium carbonate, then see what can be
distilled off under vacuum. Or, extract it with some inorganic solvent.
Not sure though. Sigma calls for -20C as a storage temp. Could be unstable.
[Edited on 17-11-2015 by Praxichys] |
The attached patent say that equimolar amounts could be used with good yields.
http://pubchem.ncbi.nlm.nih.gov/compound/2-Aminoethyl_dihydr...
melting point: 241-243 °C, about the same of patent attached ( 240-242°C).
EDIT: I found this synthesis: https://orgprepdaily.wordpress.com/?s=ethanolamine where author report about 50% yield from this reaction and says mp of product is 230-234°C.

Attachment: US3644603-aminoethyl phosphate.pdf (119kB)
This file has been downloaded 546 times
[Edited on 2-1-2016 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
MDMa is supposed to be anticancer drug. Go make some MDMA then.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
No thanks.
Actually phosphorylethanolamine is an important endogenous substance used to form cell membranes and also as a signaling agent in the body.
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
ItaBier
Harmless

Posts: 4
Registered: 17-11-2015
Member Is Offline
Mood: No Mood
|
|
The problem is how to cristalize a viscous liquid like honey.
Brazilian patent:
Attachment: PI0800460.pdf (497kB)
This file has been downloaded 829 times
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
I think it should be pretty straightfoward, just add ethanol and cool it down. You could omit the inert atmosphere too, just bubble some air in to
drive water out.
90% is just too good to believe though...
Bromine, definitely bromine.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Like the NexusDNA said, the real problem is not this, cause this liquid is just molten and crude phosphorylethanolamine. I would simply put the molten
thing in a big glass plate to cool down and then break it finely to dissolve it and recrystallise.
Note that phosphorylethanolamine is just the precursor to make the mixed salts that are composed the USP pills -> "1 mol phosphoethanolamine
neutralized by 0,42 mol CaCO3 + 0,11 mol MgCO3 + 0,06 mol ZnCO3" referenced in the USP team patent.
The method they use to neutralize the phosphoethanolamine (mixing the carbonates and the phosphoethanolamine moisting it with water and letting it
react for 5 days at 70°C) maybe can alter the crystal structure of final product and explain the higher biodisponibility of phosphoethanolamine
salts.
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I haven't had time to try it myself, but wikipedia states that a high temperature reaction between phosphorus pentoxide and sodium chloride produces
phosphoryl chloride vapor, which could be collected and used to produce the phosphate ester.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Quote: Originally posted by Amos  | | I haven't had time to try it myself, but wikipedia states that a high temperature reaction between phosphorus pentoxide and sodium chloride produces
phosphoryl chloride vapor, which could be collected and used to produce the phosphate ester. |
I wouldnt waste precious P2O5 neither phosphoryl chloride in the synthesis of phosphorylethanolamine since it can be made from
phosphoric acid, maybe you can use for another phosphoric esther that would be hard to make from plain phosphoric acid.
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
The attached doc GB1092185 is the Hans Nieper / Franz Kohler patent regarding Ca-EAP and a mix of K and Mg aspartates. It is stated that treating with
Ca-EAP alone can develop Mg and K deficiency, so the aspartates of this salts are administred along with Ca-EAP. In the end of the patent in procedure
I and II they say the simplest way how to get Ca-EAP from phosphoethanolamine.
Another attached patent US5068228 is a much more general and interesting document, as it focuses on alkylamino esters of phosphoric acid not just
phosphoethanolamine but its salts and related substances and some possible pharmaceutical uses. They make EAP salts somewhat different, as they
dissolve phosphorylethanolamine in alkaline media, add calcium hydroxide and bubble CO2 and filter calcium phosphate and carbonate. They
state a high yield (88%) although I think this way seems to hydrolise the phosphoric ester to some extent.
Attachment: fosfo-GB1092185A.pdf (241kB)
This file has been downloaded 777 times
Attachment: US5068228-usos de eap e correlatos.pdf (332kB)
This file has been downloaded 555 times
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
cacaancu
Harmless

Posts: 1
Registered: 4-7-2016
Member Is Offline
Mood: No Mood
|
|
Oi Ita. Eu consegui resolver este problema. Tens face? nos falamos in box
|
|
|