| Pages:
1
2
3 |
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
Preparation of potassium hexafluoromanganate(IV) from household items
In another thread, the preparation of fluorine was briefly discussed, particularly Karl O. Christe's preparation of fluorine without electrolysis:
Discussion of these reactions, particularly the first one, yielded the following comments: Quote: Originally posted by woelen  | | Both of Christe's reactions for making F2 without electrolysis require strictly anhydrous conditions. The first reaction hence requires anhydrous HF,
anhydrous H2O2 and a very strong drying agent to absorb the water, formed in the reaction. |
While I agreed
that the second, fluorine-producing reaction would be difficult and dangerous to attempt, the first reaction seemed quite tame. Contrary to woelen's
assertion, the original documentation suggests the reaction does proceed in aqueous solution (actually, concentrated hydrofluoric acid). Based on this, I
attempted Christie's first reaction, using dilute hydrofluoric acid, in the form of a cleaning product, as my sole source of fluoride. All other
reagents were also available as household products.

300ml of dilute hydrofluoric acid solution was added to a 500ml polypropylene beaker. The exact concentration is unknown; the MSDS states 1%-2.3%, and the quantities of other reactants were calculated assuming 1.2%, as an excess of hydrofluoric acid does not effect
the reaction. To this was added 12g of 14% potassium hydroxide solution, 4.7g potassium permanganate dissolved in a minimum of water, and 52g 3%
hydrogen peroxide.
<iframe sandbox width="280" height="160" src="//www.youtube.com/embed/PgXvecQw6yQ?rel=0" frameborder="0" allowfullscreen></iframe>
The reaction produces oxygen gas; avoid inhaling the fluoride-containing aerosol. The result is a light yellow solution/suspension.
K2MnF6 is a sparingly-soluble yellow compound, so the synthesis was successful. This liquid could be mistaken for a suspension of extremely small manganese
dioxide particles, however, once enough peroxide has been added to remove all purple/pink color, addition of additional peroxide does not produce more
gas, as would be expected from a suspension of manganese dioxide particles.
Though the above shows some fluorine chemistry can be performed with little danger, I do not take the real risks lightly. This post was intentionally
made as my 50th post, when my rank transitions to "Hazard to Self". Due to the formation of a small amount of fluoride-containing mist, this was
performed in a well-ventilated area, with googles and respirator, and the area was wiped down with limewater afterword. I will not be
attempting the second of Christie's reactions for the production of fluorine.
This may or may not be part of my entry for j_sum1's competition. 
[Edited on 19-11-2015 by MolecularWorld]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Nice job. Did you isolate the potassium hexafluoromanganate(IV)? If so, what was your yield? I'm curious to see if using such a dilute solution of
HF decreases yield significantly from the yield in the original paper. It's quite possible that the yellow solution arose from another manganese
compound than K<sub>2</sub>MnF<sub>6</sub>, so I would recommend verifying that you actually synthesized what you thought you
did.
[Edited on 11-19-2015 by gdflp]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You're going to have to do a whole lot better than a 1 min UToob to convince me you've prepared K2MnF6. Like maybe a little elemental
analysis, you know?
You do realise KMnO4 oxidises peroxide easily, right?
| Quote: | | K2MnF6 is a sparingly-soluble yellow compound, so the synthesis was successful. |
Talk about an aversion to evidence...
[Edited on 19-11-2015 by blogfast25]
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
@gdflp: I did not isolate the compound, and destroyed the solution with calcium hydroxide. The only ways I know to isolate the compound from such a
dilute solution would require very large amounts of acetone, or evaporating 500ml of hydrofluoric acid. I may repeat the procedure once I feel I can
perform either isolation, and measure the purity of the product.
@blogfast: Good thing I wasn't trying to convince you! Just for fun, what do you think the reaction in the video was? I assure you, the reagents were
exactly as I described. What else could account for the color change, and gas production, which ceased once the solution had turned light yellow (I
added more peroxide after the end of the video)?
I currently have only the most limited means for analysis, the results of which surely wouldn't meet your standards. Though I may repeat the procedure
in the future once my abilities have increased.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
You could also try making more concentrated HF from a fluoride salt, though be very cautious if you do decide to do that, of course.
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zts16  | | You could also try making more concentrated HF from a fluoride salt, though be very cautious if you do decide to do that, of course.
|
I do not plan to attempt any further fluorine chemistry, other than repeating the procedure above once I can better analyze the products. The purpose
of this experiment was merely to see whether the desired complex could be produced from dilute solutions. I believe I provided enough evidence for
this to be plausible, though blogfast is correct that my proof is far from absolute.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I can see that I am going to have to look at this a whole lot more closely. I have always assumed that fluorine chemistry was almost completely out
of reach for the normal person. Maybe you are not normal, MW. This all looks pretty exciting.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
It would appear that in the US nearly every reagent possible is OTC, as long as it contains 1% detergent 
A very nice idea MolecularWorld, but I must admit that I too am somewhat sceptical.
I suggest you perform this reaction side-by-side with an acidified control of similar volume and concentration but minus the fluoride cleaner.
Take care to match the two as closely as possibly, the one simply lacking the small amount of HF.
I think the principle doubt here is whether highly diluted hydrofluoric acid is capable of dissolving the formed MnO2 suspension at such dilute
concentrations and so a control would be most convincing.
Finally, you might also want to add an excess of your dilute hydrofluoric acid and see if you can't dissolve the suspension completely as a solution
of MnF6(2-)(aq).
[Edited on 19-11-2015 by deltaH]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
This is not K2MnF6. The salt is decomposed by water and you are working in nearly pure water with neglectible concentrations of reagents. The whole
procedure is different from your experiment. And the "original documentation" is a different one. This:

http://onlinelibrary.wiley.com/doi/10.1002/ange.19530651108/...
| Quote: | | K2MnF6 is a sparingly-soluble yellow compound, so the synthesis was successful. |
You did't get a precipitate, so your conclusion is wrong.
| Quote: | | This liquid could be mistaken for a suspension of extremely small manganese dioxide particles, however, once enough peroxide has been added to remove
all purple/pink color, addition of additional peroxide does not produce more gas, as would be expected from a suspension of manganese dioxide
particles. |
Only MnO2 and K2MnF6 can be produced? You are working in a diluted acidic medium! Your neither get one of these products but simply MnF2 which
explains your observation that additional H2O2 isn't decomposed. The yellow colour could be anything, probably side products.
[Edited on 19-11-2015 by Pok]
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
@Pok: Thanks for your input. I can't translate your German text above, but I assume from your comment it stipulates anhydrous conditions are required
for this reaction. This contradicts with the paper I cited above, which states: | Quote: | | The salt selected for this study was K2MnF6. It has been known since 1899 and is best prepared from aqueous HF solution.
|
As for the lack of precipitate, you know I'm working with very dilute reagents, and not nearly enough HF concentration to force the complex to
precipitate, and the lack of precipitate is still surprising? I'll see what can be done to extract the solid compound from this solution.
And finally, MnO2 and K2MnF6 are the only compounds/complexes that could be formed in this mixture that are yellow,
that I know of. You say the yellow must be a side product, but don't state what it could be.
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Take a look at the references of your source. Your source quotes my source. And your source doesn't specify the reaction conditions. Anhydrous
conditions aren't necessary, but highly concentrated solutions - e.g. 30 % H2O2 instead of 3 %, 40-50 % HF instead of 1-2 %. H2O2 has to be added drop
by drop. The reaction has to be cooled with ice.
Here is a similar instruction from Christie's description:
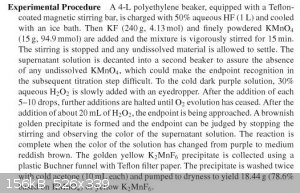
http://onlinelibrary.wiley.com/doi/10.1002/9781118409466.ch1...
The lack of a precipitate isn't surprising. But your observation is only based on a colour and nothing else! There is not the slightest evidence for
the existence of the desired product.
What the yellow stuff could be? I stated it: impurities. You used household chemicals. These aren't pure reagents but can contain all sorts of
contamination. It's impossible that it is K2MnF6, because that hydrolyses in water and your whole procedure lacks in some essential conditions.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Oh hey POK  Nice to have at least one other VC member here. Nice to have at least one other VC member here.
There is a short passage on that compound in "The Chemistry of Manganese, Technetium and Rhenium".
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
@Pok: Interesting. Based on your reference, I will attempt the procedure again, in an ice bath and with ice-cold solutions, to see if a precipitate
forms. I'm also considering forcing the compound to precipitate with a large quantity of acetone, although I'm slightly concerned with the potential
formation of acetone peroxide.
The yellow could be from impurities in the reagents, but I doubt it. Three of my four reactants were perfectly colorless solutions, and the
permanganate is sold for use in iron-removing drinking water purification devices, so it's unlikely to contain other transition metal impurities.
[Edited on 19-11-2015 by MolecularWorld]
|
|
|
softbeard
Hazard to Self
 
Posts: 69
Registered: 23-7-2013
Member Is Offline
Mood: moody
|
|
MolecularWorld, I think you should be careful before making statements like "K2MnF6 is a sparingly-soluble yellow compound, so the synthesis was
successful".
Anyone with a chemistry background would know that it's impossible to synthesize K2MnF6 under the conditions you're describing.
But even leaving that aside, you can't confirm a compound's identity by wishful thinking and because it has a colour similar to your desired compound.
Have fun & take care with the fluoride experiments. I would suggest giving up on producing elemental fluorine for now. I think copper chemistry is
a lot more fun and colourful!
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by softbeard  | MolecularWorld, I think you should be careful before making statements like "K2MnF6 is a sparingly-soluble yellow compound, so the synthesis was
successful".
Anyone with a chemistry background would know that it's impossible to synthesize K2MnF6 under the conditions you're describing.
But even leaving that aside, you can't confirm a compound's identity by wishful thinking and because it has a colour similar to your desired compound.
|
Clearly. The more experienced members here will settle for nothing less than mass spectrometry and x-ray crystallography to confirm the identity of a
compound. I drew my conclusion from the fact that there's a very limited number of yellow products that could be produced from these reactants, and
the fact that my synthesis is similar (though not identical) to that which was reported in the literature. As Pok pointed out, and I agreed, this
evidence is far from absolute. But, it's not just "wishful thinking". Compare, for example, all the preparations of tetraamminecopper(II) complexes
reported on this board, where color is used as the primary, and sometimes only, evidence of reaction.
| Quote: | | Have fun & take care with the fluoride experiments. I would suggest giving up on producing elemental fluorine for now. I think copper chemistry is
a lot more fun and colourful! |
In the very first post:
Followed by: Quote: Originally posted by MolecularWorld  | | I do not plan to attempt any further fluorine chemistry, other than repeating the procedure above once I can better analyze the products. The purpose
of this experiment was merely to see whether the desired complex could be produced from dilute solutions. I believe I provided enough evidence for
this to be plausible, though blogfast is correct that my proof is far from absolute. |
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
It indeed is wishfull thinking. You see what you want to see. Nothing else. Thousands of colourless chemicals can form yellow/brown products by
reacting with KMnO4. Well, one thousand still is a "limited number". 
There is no need for spectroscopy to check the yellow stuff. K2MnF6 hydrolyzes in water. It's simply impossible to make a solution of this material in
1-2 % HF!
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
Name three yellow products formed from the reaction of a colorless solution with potassium permanganate, not including potassium
hexafluoromanganate(IV) and manganese dioxide. Be sure to take my comment above about likely impurities (ie, there wouldn't be other metals in the
permanganate, or heavy metals in medical-grade peroxide) into consideration.
The comments here, particularly yours (Pok), have convinced me that my product might not be potassium hexafluoromanganate(IV). But this has
yet to be proven definitively. Rather, my baseless assertions have been countered by... baseless assertions. My hypothesis is that the main reason
concentrated hydrofluoric acid is used in the literature preparations is to precipitate the complex before it can hydrolyze, but that small amounts of
unstable potassium hexafluoromanganate(IV) could form in dilute solution/suspension. At least I conducted an actual reaction; if someone repeats my
procedure, and proves the yellow stuff produced is not potassium hexafluoromanganate(IV), then I'll be convinced.
[Edited on 19-11-2015 by MolecularWorld]
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
Here's an excerpt from the reference fluorescence mentioned. My wishful thinking has me focused on the keyword "slow".

[Edited on 19-11-2015 by MolecularWorld]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Why don't you carry out the simple control experiment I suggested?
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
This one?
Quote: Originally posted by deltaH  | | I suggest you perform this reaction side-by-side with an acidified control of similar volume and concentration but minus the fluoride cleaner.
Take care to match the two as closely as possibly, the one simply lacking the small amount of HF. |
I'll do it, although I don't fully understand what it's intended to prove.
Which acid would be suitable as a substitute for the HF? HCl is liable to react and release chlorine, would sulfuric acid do?
[Edited on 19-11-2015 by MolecularWorld]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
H2SO4 should be fine.
[Edited on 19-11-2015 by deltaH]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Not "slow" is the keyword but "even in HF solution"! Again the original literature:

https://books.google.de/books?hl=de&lr=&id=DbACnFyLS...
So you see that your source simplified the main statement from the original source!
In "aqueous solution" can mean anything but it doesn't necessarily mean "in water". 50 % HF is an aqueous solution, too. If you look at the
decomposition products: Mn(III) ions will form. These are yellow/brown in solution as far as I know.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Speaking of which, I suspect the precipitate is a suspension of hydrated Mn2O3 or manganese oxyhydroxide, exactly why the control is needed. You
should see a similar result.
Here's why:
Attachment: Pourbaix_diagram_for_Manganese.svg (52kB)
This file has been downloaded 614 times
[Edited on 19-11-2015 by deltaH]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Suspensions of very fine, freshly precipitated MnO<sub>2</sub> appear yellow. See: the final state of the 'chemical chameleon' reaction
(see my blog here). The final picture is a bit on the orange side, but most times I've done this reaction it ends at a nice pale yellow.
People's reaction to your experiment has been a bit harsh, in my opinion, but they do make a valid point. Color alone isn't definitive when looking at
a reaction product, though it certainly can be one point of evidence. You keep challenging others to come up with a possible yellow product, but it's
nearly impossible for anyone to say what it could be because you're using household chemicals. Who knows what impurities are present? From my
experiment above, only very small amounts of chemicals led to very visible colors. Maybe some detergent or inactive ingredient in your cleaners
reacted in some way. There's no way to tell without knowing exactly what's in there (and the MSDS's aren't always all-inclusive, either).
Clearly no one's expecting someone at home to utilize state-of-the-art analysis equipment, but one or two more pieces of evidence are necessary before
we can claim with certainty that you got what you think you got. If you were able to isolate the colored compound and run some more tests on it, that
would help greatly. For example, when I made Chevreul's Salt, the color was pretty indicative but I went ahead and ran some extra tests to show it contains both copper(I) and copper(II).
I thought this thread was all pretty interesting, for what it's worth.
Edit: Speaking of "tetraamminecopper(II) complexes," I've made hexaaminenickel(II) chloride before (it's on my YouTube Channel). Near the end of the video, you can see that some leftover on the filter paper turned from purple to green upon drying in air. I
wasn't sure at the time, but I've since learned that if it's left out, the ammonia leaves the complex and it reverts to normal hydrated nickel(II)
chloride. So an ammonia smell of the purple compound vs. no smell of the green compound is another indication I made what I think I did.
[Edited on 11-19-2015 by MrHomeScientist]
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
@MrHomeScientist: I was well aware that the solution could be a fine suspension of manganese dioxide, which is why I tested it with additional
peroxide, as was already noted in my original post and later posts. Additional peroxide did not decompose, so it's (probably) not manganese dioxide.
I don't mind harsh, though I do mind harsh-and-useless, like blogfast's comment above. I very much appreciate Pok providing detailed explanations and
references, in addition to voicing doubt. You and Pok are technically correct in that the yellow product 'could be anything' as the contaminants
'could be anything', though I've also explained above how unlikely it is that these products are contaminated with anything that could give a colored
product. I've been researching it, and the only possibility I've found is if my drain-opener potassium hydroxide was contaminated with lead(II)
hydroxide, it could form yellowish lead(II) oxide, but really, how likely is that?
@deltaH: I believe Mn2O3 would also decompose hydrogen peroxide. If so, per my above testing, it's not that.
@Pok: I'm probably misunderstanding things, but that would seem to confirm that the desired complex could form, then hydrolyze. Just because I
couldn't (yet) extract and purify the compound, doesn't mean it didn't form. Also, you almost had me convinced that the resulting solution contains a
fluoride of manganese, but both MnF2 and MnF3 are pink. Since it's not manganese dioxide, and not a fluoride of manganese, where
did the manganese go?
Despite the apparent futility, I'll be attempting to purify a solid compound from [a fresh batch of] the solution prepared by my above procedure. I'll
also do deltaH's 'control' reaction.
[Edited on 20-11-2015 by MolecularWorld]
|
|
|
| Pages:
1
2
3 |