gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
N-isopropyl-2-propanimine preparation
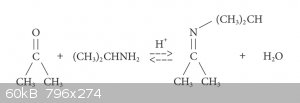
I've been planning to make this for a while now using the classic imine synthesis route shown in attachment.
N- isopropyl-2-propanimine is formed via the reaction of acetone with isopropylamine, both of which I have. The reaction is an equilibrium as the
water and imine react to form the starting materials, and it requires an acid cataylst. To tip the equilibrium in favour of the imine, the water must
be removed from the reaction as soon as it's formed. I plan to use dichloromethane as the solvent for the reaction, since water is imicsible in it.
My synthesis plan is shown below:
First, 43ml of isopropylamine and 100ml of dichloromethane are added to a large beaker. Next, 35ml of acetone is added along with the acid catalyst.
The mixture is left at room temperature overnight and after this, an upper aqueous layer should form. The bottom organic dichloromethane is extracted
using a pipette and placed in a round bottom flask. Distillation apparatus is attached to the round bottom flask and dichloromethane is distilled off
at 40 C leaving the imine product which boils at 92.5 C.
I have a few questions:
1 Is this procedure likely to work?
2 What's an ideal acid cataylst to use that doesn't form a salt with the amine?
3 The only information I can find on N-isopropyl-2-propanimine is its boiling point (92.5 C). Is it likely to be extremely dangerous?
Thanks in advance, kind humans 
[Edited on 21-12-2015 by gluon47]
reality is an illusion 
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
Imines can form in DCM, but it will not be quantitative. If you want to be sure of imine formation you would have more success using toluene. Make a
solution of the two in toluene, and distill off the water-toluene azeotrope to form the imine in quantitative yield.
You can check that you have removed all of the water if you have a dean stark apparatus, but failing that you can just add fresh toluene and keep
distilling it off to the same effect. You can't over do it.
This has the nice advantage of giving you the pure imine, free from any salts.
[Edited on 21-12-2015 by User123]
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
thanks user! I'll try what you suggested once I've got everything prepared. does it not require an acid catalyst?
reality is an illusion 
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
No, it doesn't. The removal of water as an azeotrope will drive the reaction forward on its own.
It's a generic method, I've prepared many different imines using this method.
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
great. cheers for the help . can't wait to try this. . can't wait to try this.
reality is an illusion 
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
Thinking about it, you may need to reflux it for a little bit before distilling it off, to give the acetone some time to react before it's distilled
off.
Perhaps it didn't need saying, but anyway...
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
Quote: Originally posted by User123  | Thinking about it, you may need to reflux it for a little bit before distilling it off, to give the acetone some time to react before it's distilled
off.
Perhaps it didn't need saying, but anyway... |
I'll take that into account. thanks again.
reality is an illusion 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Do you need to prepare this exact imine, or just any imine? Do you need to start from acetone and isopropylamine?
It would be much easier to prepare this imine from diisopropylamine using one of the oxidative methods. Even trichloroisocyanuric acid can be used as
the oxidant and you can use excess diisopropylamine as the required base. (If you are desperate, you can prepare diisopropylamine from acetone and
isopropylamine, though diisopropylamine is very cheap and generally easily available.)
To prepare it from acetone and isopropylamine you would need to apply the standard TiCl4 method, see DOI: 10.1021/jo01285a088 and also Acta Chem. Scand., 1992, 46, 1211. It may not be particularly home chemist friendly.
If you just want to make any imine, then make a benzylidene amine. They are terribly easy to prepare. You just stir your primary amine with your
benzaldehyde of choice in ethanol, methanol or even neat if at least one reactant is liquid. The reaction usually takes place in minutes for most
benzaldehydes, utmost an hour or so for the less reactive combinations. Some crystallize directly from methanol if concentrated enough. Others might
require evaporation and recrystallization.
Quote: Originally posted by User123  | No, it doesn't. The removal of water as an azeotrope will drive the reaction forward on its own.
It's a generic method, I've prepared many different imines using this method. |
Except that it is not a general method and it will not work in this specific case for obvious reasons.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
The reasons are clearly not obvious to me, so perhaps you could make them so?
As for it not being a general method: I have used it generally and it has worked generally. Given that there are an infinite number of amines and
aldehydes and ketones, however, it is entirely possible that what I have observed to work in general, you have observed not to work 'in general'. And
so generally speaking, I was wrong to generalize.
Although, if you apply that logic, there are enough exceptions to EVERY reaction that you could say that literally NOTHING generally works. I suppose
that is the fundamental truth of the matter.
However, azetropic removal of water to form imines is common enough to be described in many practical organic chemistry textbooks, so I maintain that
it IS a general method of forming imines. I suspect that your experience is highly atypical, if you think that it is not. Which suggests that you are
working on more interesting chemistry than most of us, where we can simply form imines by removing water mechanically.
[Edited on 22-12-2015 by User123]
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
User123. What's the mechanism for carbinolamine dehydration w/o an acid cat.? This probably isn't problem Nicodem sees (which is not obvious to me
either), I'm just curious.
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
I don't know anything about mechanistic studies of uncatalysed imine formation. Here's an example of a reaction which proceeds without an acid
catalyst, though, if you need convincing that it is even possible: http://www.orgsyn.org/demo.aspx?prep=CV1P0080
[Edited on 22-12-2015 by User123]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Zyklon-A, you do not need an acid for the elimination step. The acid just catalyzes that elimination. It will proceed in the absence of any acid as
well, particularly in the reaction with benzaldehydes (for reasons that should be obvious), or even in ketimines synthesis upon heating in refluxing
toluene (though for practical reasons a few mol% of TsOH or some other acid is commonly added). Also, observe that hemiaminals can also undergo an
E1cB elimination, which in their particular case requires no basic catalysis (see their electronic structure).
Note also that when forming corresponding imines from nucleophiles having an alpha effect, like oximes from hydroxylamines or hydrazides and azines
from hydrazines, no acid is used, indicating that it is the nucleophilic addition step that is the rate determining.
The reason why such a strong acid like TiCl4 is used in the cited method is not so much to catalyze the elimination step, but activate the
ketone carbonyl group and sequester the formed water. Note that an excess of triethylamine is used in relation to TiCl4, hence its acidity
is actually quite attenuated and the true reagent is the Et3N.TiCl4 complex.
Obvious, as in the sense that it obviously not possible to azotropically remove water from a mixture of acetone (bp 56 °C) and isopropylamine (bp 34
°C) using toluene.
I have used this azeotropical method a few times to form the enamines from aryl alkyl ketones and secondary amines. It worked somewhat, but it took at
least two days of reflux to achieve conversions above 70% (and using TsOH did not help much). Obviously I wasn't very satisfied with it, but it was
still more practical than using the TiCl4 method, because I could use the ketimine solution for the next synthetic step as such. It might
work faster with primary amines giving imines, but as far as I know it sucks for the synthesis of the less reactive aliphatic ketimines and I never
heard of anyone being satisfied with the results applying such substrates.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
Hence I advised him to reflux the toluene to form the imine, then distill the toluene to dry the IMINE.
Please read more carefully.
He wants to prepare a pure, dry imine.
This was made plain. I even said that it 'hardly needs saying', because it was so obvious. Yet for you, it needs REITERATING.
Now it has been.
The chap understood perfectly what I said.
Perhaps you could try reading less literally in future. Assume that people aren't morons.
[Edited on 22-12-2015 by User123]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by User123  | Hence I advised him to reflux the toluene to form the imine, then distill the toluene to dry the IMINE.
Please read more carefully.
He wants to prepare a pure, dry imine. |
But he is trying to prepare an aliphatic ketimine, the reaction mixture will be refluxing at no more than 40-50 °C, and all water will be
returning back with the isopropylamine/acetone condensate thus maintaining the same initial equilibrium point constant. So what would the point of
such treatment be?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
Then he should use IPA.
The purpose of refluxing is to form the imine. It's a simple enough operation. Why make out that it's so bloody difficult?
You could mix them in methanol and you'd have a 90 plus % yield in about 5 minutes.
The purpose of the toluene is to get rid of the water.
All this fucking chat over forming an imine. Jesus Christ.
[Edited on 22-12-2015 by User123]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by User123  | Then he should use IPA.
The purpose of refluxing is to form the imine. It's a simple enough operation. Why make out that it's so bloody difficult? |
I'm not sure you noticed, but he is trying to prepare an aliphatic ketimine.
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
@nicodem, to answer your original question, no I don't have to make this particular imine. I just thought it would be the easiest one to make with the
resources available to me. However i have become rather fond of it and would like to make it if possible. I'll probably try benzylidene amines as well
as they sound fairly easy to make from what you described.
You suggested making N-isopropyl-2-propanimine via the oxidation of diisopropylamine. Where could I find more information on this?
I'll try user123's idea as well.
Thanks everyone for your suggestions. Sorry if I'm missing something. I don't want to make any enemies.
[Edited on 22-12-2015 by gluon47]
reality is an illusion 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gluon47  | | @nicodem, to answer your original question, no I don't have to make this particular imine. I just thought it would be the easiest one to make with the
resources available to me. However i have become rather fond of it and would like to make it if possible. I'll probably try benzylidene amines as well
as they sound fairly easy to make from what you described. |
I still think you should first practice on trivial imines such as oximes or N-benzylidene amines. Simple water sensitive imines are difficult to
handle and you can't do them on a small scale as the isolation requires fractionation on a distillation column. It can be quite involved in regard to
experience and required equipment and at the end you obtain a liquid product for which you need spectroscopic or GC-MS analysis. Solid products are
much easier to characterize in a home chemistry lab, as all you need is do a TLC and measure the mp.
| Quote: | | You suggested making N-isopropyl-2-propanimine via the oxidation of diisopropylamine. Where could I find more information on this?
|
One home chemist friendly example employing trichloroisocyanuric acid is described in DOI: 10.1055/s-2004-830896 (the article is available from this
forum, UTFSE). It takes oxidizing excess diisopropylamine in dichloromethane. You can then remove the insoluble cyanuric acid by filtration and
separate the imine from the diisopropylamine hydrochloride by distillation.
Other oxidation methods in the literature are not particularly trivial, like oxidation with air catalyzed by gold (DOI: 10.1039/B700555E) and other
more demanding ones.
Perhaps the most industrially friendly route is described in WO2003042158 (gas phase reaction of isopropylamine with propyne). It is also among the
most unfriendly methods for non-industrial chemists. Several other more or less exotic methods are described in the literature, but not really worth
mentioning, particularly since the imine is rarely isolated or the yields are low.
Only one direct condensation between isopropylamine and acetone is described in the literature (DOI: 10.1055/s-1997-1211). If it works well, it might
be almost as practical as the oxidation route, just takes some more work and time, but it should be better scalable (it is already reported on a molar
scale). It is as simple as drying the neat reaction mixture, which was firstly equilibrated in the presence of a few mol% HCl, over NaOH and then
fractionation by distillation from more NaOH. It is claimed to give a 67% yield of the imine. I'm surprised it works as this usually does not work on
just any ketone and is most commonly used on cycloaliphatic ketones, but looks like acetone is after all reactive enough and NaOH is a strong
dehydrating reagent (sterically undemanding, I guess). I suggest you try this out and post about your experience. Even if it turns out the reported
yield is unachievable, the method still has an option to insert one or two more stages of dehydrations over NaOH to increase yields.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
Thanks nico. Most of those procedures look feasible.
Quote: Originally posted by Nicodem  |
I still think you should first practice on trivial imines such as oximes or N-benzylidene amines. Simple water sensitive imines are difficult to
handle and you can't do them on a small scale as the isolation requires fractionation on a distillation column. It can be quite involved in regard to
experience and required equipment and at the end you obtain a liquid product for which you need spectroscopic or GC-MS analysis. Solid products are
much easier to characterize in a home chemistry lab, as all you need is do a TLC and measure this. |
I'm sure your right. I will have a go at making a N-benzylidene amine before i attempt to prepare N-isopropyl-2-propanimine.
reality is an illusion 
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Me, I got no use for said imine. Though it might be easily reduced to di-isopropylamine, a fairly expensive and not so easily obtained reagent.
In which case, the imine could probably be reduced as formed, either by hydrogenation, or possibly a dissolving metal reaction....such as reduction by
Aluminum Amalgam.
PS. Whoops! I stand corrected. A quick online search reveals that Di-isopropylamine, is not currently very expensive. If it is also easily
obtained, I am twice wrong. Though it might still be fun to make some.
[Edited on 27-12-2015 by zed]
|
|
|