CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
MEK---->DEK Conversion?
I recently found some information online about a reaction process called the Wolff-Kishner Reduction. The reaction goes about converting carbonyl
functionalities into methylene groups:
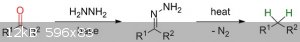
In theory,the reaction can be used to convert acetone and methyl ethyl ketone to propane and butane respectively. This got me thinking: What about
converting diethyl ketone (2-Pentanone) to what I read to be pentane, with the formula C5H8? Neither pentane nor diethyl ketone
are sold on the Internet,so I am wondering if there is any way to convert methyl ethyl ketone to diethyl ketone,or any way to convert acetone to DEK.
While I am discussing this,how would the generation of propane,butane,or pentane go? What would a "generator" setup look like? What I currently have
planned is to add a mixture of "dried" hydrazine(I'm sure it has been discussed on the forum before,so I won't go into much detail as to obtaining it)
and sodium hydroxide to acetone or other ketone,then heating it to 90C, I am unsure of what to do next,so any advice would be welcome.
[Edited on 23-12-2015 by CitricAcid]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
"...convert methyl ethyl ketone to diethyl ketone,or any way to convert acetone to DEK."
No. Not in any reasonable way that I know of.
If you want propane, buy it, it comes in 20 lb tanks. As for butane, likewise, but in smaller cylinders. As for pentane, distill some gasoline or go
for pet ether.
"Dried" hydrazine sounds nasty. I'd avoid that. Other routes involve the Clemmensen reduction with HCl and Zn, preferably amalgamated. This is
probably your best bet if you must do this. Neither the substrate nor the product are particularly sensitive, so the other option (neutral) of
thioketal desulfurization (treatment with dithioglycol aka dithioethane, followed by reduction with Raney nickel) is ridiculous.
Anyhow, unless this is for your edification (which is OK!), I see no less circuitous route than simply buying or refining the alkane you want.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ozone  | "...convert methyl ethyl ketone to diethyl ketone,or any way to convert acetone to DEK."
No. Not in any reasonable way that I know of.
If you want propane, buy it, it comes in 20 lb tanks. As for butane, likewise, but in smaller cylinders. As for pentane, distill some gasoline or go
for pet ether.
"Dried" hydrazine sounds nasty. I'd avoid that. Other routes involve the Clemmensen reduction with HCl and Zn, preferably amalgamated. This is
probably your best bet if you must do this. Neither the substrate nor the product are particularly sensitive, so the other option (neutral) of
thioketal desulfurization (treatment with dithioglycol aka dithioethane, followed by reduction with Raney nickel) is ridiculous.
Anyhow, unless this is for your edification (which is OK!), I see no less circuitous route than simply buying or refining the alkane you want.
O3 |
I want to perform the Wolff Kishner reaction just for the sake of doing it,not because I need propane/butane/pentane for any purpose. Yes,I do want
to go with the hydrazine route. Once I got an idea of the complete setup for the reaction,I am planning on using a dry ice CO2 generator to hopefully
prevent an explosion of the hydrazine(Makeshift inert atmosphere?). If you know where to get diethyl ketone or have any ideas for the procedure after
converting the ketone to hydrazone,I would appreciate it if you told me.
[Edited on 23-12-2015 by CitricAcid]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Why go through the hydrazone when other less-explosive routes exist?
And, no, diethyl ketone isn't that common. That is, it's not a common industrial solvent. It's about $300 (US) for 2.5 L (about twice as much as MEK,
Sigma), which isn't ridiculously expensive, but it's not cheap, either.
But, why bother, butane can be had from MEK, this way, and 98+% MEK can be had from paint/home improvement stores.
So, then, why DEK, in particular?
Oh, you will also have to work in a pressure-rated vessel (one way or another), so there's that.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ozone  |
Oh, you will also have to work in a pressure-rated vessel (one way or another), so there's that.
O3 |
What is the minimum required pressure rating if I were to go through and do this using borosilicate glassware?
I don't want to have to bring the setup to 45 psi (Random amount)when the glassware can only handle 30 psi(Random amount,I don't know how much
pressure Pyrex brand glassware can actually handle).
[Edited on 23-12-2015 by CitricAcid]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
You want to collect a liquified alkane, do you not? Neither propane nor butane (pentane is, barely) are liquid at room temperature (what will
inevitably occur after whatever cryo is used for condensation) and atmospheric pressure.
Look it up.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
I figured he wanted to just run it through a bunsen for proof-of-concept. Keep in mind it will contain large amounts of nitrogen.
I think a more interesting substrate would be cinnamaldehyde, and I hear WK can be done with N2H4 sulfate and KOH. Don't know how well the unsaturated
aldehyde would play with KOH, retro-aldol and all that.
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ozone  | You want to collect a liquified alkane, do you not? Neither propane nor butane (pentane is, barely) are liquid at room temperature (what will
inevitably occur after whatever cryo is used for condensation) and atmospheric pressure.
Look it up.
O3 |
As Stygian said,I am going to burn off the propane/butane to confirm success and won't bother liquefying it.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Then it doesn't matter. But, it's a lot of trouble to go through without having a "product in the bag."
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
I already looked up what a WK would convert cinnamaldehyde to,and the product is most likely allylbenzene. The MSDS that I have looked at so far don't
say anything about lethal respiratory exposure,only oral data. Hell,the only other important data available are the molecular weight,specific gravity,
autoignition point,boiling point,and flash point.
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Before the entirety of this thread gets discarded to Detritus,I have one last question to ask:
Let's suppose I actually went through with the setup. Would adding a mixture of hydrazine or hydrazine sulfate and sodium or potassium hydroxide to
the desired ketone,heating the mixture,and then adding a solvent like ethylene glycol and then potassium hydroxide,then heating that result in the
final methylene product?
|
|
|
Texium
|
Thread Moved
22-12-2015 at 21:27 |
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
You can buy pentane OTC from craft shops. It is the single largest component of gasoline.
I would not encourage using a highly toxic reagent like hydrazine to produce boring alkanes unless you have a very good reason for doing so.
|
|
|
MeshPL
Hazard to Others
  
Posts: 329
Registered: 20-4-2015
Location: Universe
Member Is Offline
Mood: No Mood
|
|
A doable MEK -> DEK conversion:
1. Run haloform reaction on MEK. One of the products is propanoic acid. Youtuber Nurdrage made 2 videos on it, using rather basic chemicals. You need
chlorine bleach, MEK and some basic chemicals for extraction of product. The other product is chloroform, unless you substitude chlorine bleach with
bromine or iodine which also work yielding respective haloform.
2. Turn propanoic acid into calcium propionate, pyrolyse it, DEK is one of pyrolysys product.
Alternatively:
2. Run vapours of propanoic acid through suitable catalyst tube. Manganese oxide or thorium oxide catalysts may work, or at least do for simmilar
reactions.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Pyrolysis of calcium Propionate lead to MEK(Methyl ethyl ketone) as main product.
Diethylketone(3-Pentanone) is one of the ketone in Calcium butyrate Pyrolysis
[Edited on 26-12-2015 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have been considering doing a Wolff-Kishner reduction myself, just for fun: ethylbenzene from acetophenone. I would use the Huang-Minlon version as
shown in Vogel.
Another interesting synthesis might be the conversion of cyclohexanone to cyclohexane via a Wolff-Kishner.
[Edited on 26-12-2015 by Magpie]
[Edited on 27-12-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Ah, OK! Huang-Minlon for the win. Hydrazine hydrate is much more forgiving. I saw "dry hydrazine" and thought "anhydrous hydrazine", then thought
"Dear God."
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
MeshPL
Hazard to Others
  
Posts: 329
Registered: 20-4-2015
Location: Universe
Member Is Offline
Mood: No Mood
|
|
Waffles SS said:
| Quote: |
Pyrolysis of calcium Propionate lead to MEK(Methyl ethyl ketone) as main product.
Diethylketone(3-Pentanone) is one of the ketone in Calcium butyrate Pyrolysis
|
This book https://books.google.pl/books?id=lg2r9BDlbM8C&pg=PA519&a... states that pyrolysys of calcium butyrate yields 4-heptanone (dipropyl ketone).
Analogically, pyrolysis of calcium propanoate should yield DEK. Pyrolysis of calcium acetate yields acetone.
|
|
|