| Pages:
1
..
45
46
47
48
49
..
60 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
if it does sink, and if this is right :
then if done in a crucible, the aluminium would protect the P from air.
Adding a hairdryer is a nice touch.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Quote: Originally posted by ScienceHideout  | Are you sure that that is correct, bromic acid?
The delta H of AlP is negative.
When we are talking about the reaction with LIQUID aluminum, the delta S is certainly negative, because we have a liquid turning into a solid
(m.p.=2530 C)
If both are negative, doesn't that mean that the enthalpy only drives the reaction, and the reaction is only favorable at low temperatures?
|
Then how about :
<s>float</s>
<s>sink</s>
<s>react</s>
<b>distill</b>
Although Hittorf's Violet is recrystallized from lead, that occurs at even lower temperatures than the melting point of aluminum. Some phosphorus
might stay behind in the aluminum melt but as it cools then I would wager that the phosphorus would react.
Admittedly I did not take the time to consider the known thermal instabilities of metallic phosphides when making my previous comment.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Reaction Delta H = -166.5 kJ/mol
I don't have specific data for the entropy.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Looks likely.
But then, Distill is good isn't it, as in do-able ?
|
|
|
jsc
Hazard to Self
 
Posts: 65
Registered: 16-3-2011
Member Is Offline
Mood: No Mood
|
|
Phosphorus.—This is obtained by heating phosphoric acid with carbon, or bone-ash or mineral phosphates with carbon and silica. In this way the
phosphorus is liberated from the compound containing it, and is distilled and condensed outside the furnace. As this operation must be carried on in
the absence of air, the electric furnace is particularly suitable, and has practically replaced all others.
The old method of making phosphorus consisted in heating bone-ash (calcium phosphate) with sulphuric acid, thus obtaining phosphoric acid or calcium
metaphosphate. This was mixed with charcoal and heated in clay retorts, phosphorus being liberated as a vapor and condensed under water.
Bone-ash or natural calcium phosphate can be smelted directly (without the sulphuric-acid treatment) to yield phosphorus, if silica is added to
combine with the lime, as well as charcoal to reduce the oxide of phosphorus, but this process could not be carried out until the introduction of the
electric furnace, as the temperature required was higher than could conveniently be obtained in a retort. At the present time, bone-ash, or the
minerals rock-phosphate, apatite, or wavelite, are mixed with silica and charcoal, and heated in an electric furnace, yielding phosphorus vapor,
carbon monoxide and a liquid slag of calcium silicate. In some cases, however, the sulphuric acid process is still employed as a preparatory step.
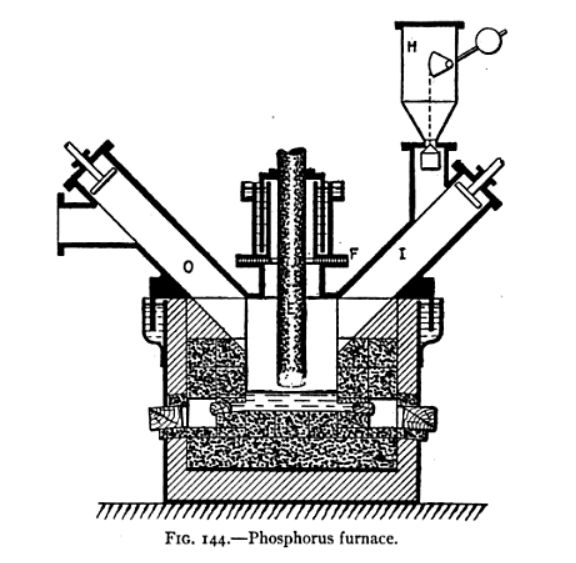
A suitable electric furnace is that invented by G. C. Landis, and shown in Fig. 144. It consists of an iron box lined with vitrified bricks and having
an inner lining of carbon blocks which form one electrode; the other electrode, E, being vertical. The charge of phosphate, carbon and silica is
introduced by means of the hopper H and inclined tube J. The phosphorus vapor and accompanying gases pass out by the pipe O to suitable condensers,
and the fused slag is tapped out below. The furnace is made gas-tight with the aid of water-seals for the cover and for the gland admitting the
electrode; the latter being insulated from the furnace body at the point F. The vitrified bricks lining the furnace are laid in a nonabsorbent mortar
such as a mixture of silicate of soda and powdered asbestos; this being intended to prevent absorption of the phosphorus. The carbon lining is
composed of two layers of blocks, so that the inner set of blocks can be replaced, when worn away, without disturbing the outer layer. The inner
tapping hole for the molten slag is plugged with a piece of wood, without any luting; an outer plug, which is luted, prevents access of air to the
inner wooden plug. Two sets of tapping holes are provided so that one set can be used when the other has become worn out.
About 80 per cent. or 90 per cent. of the phosphorus in the rock is extracted by this process, and the yield has been stated as 3.3 lb. of phosphorus
per horse-power day.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Superb !
Eagerly awaiting the photos of it in action in your backyard.
[Edited on 13-5-2014 by aga]
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
This may have already been answered but I couldn't find it.
Can Sulfur be oxidized to SO2 by P2O5 resulting in Phosphorus ?
If so it is likely that the reaction me proceed at a lower temperature vs Carbon.
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
Litmus
Harmless

Posts: 3
Registered: 28-2-2014
Member Is Offline
Mood: mad
|
|
Motherload, I couldn't say for sure but you may have something there. On the face of it, it seems reasonable to me the acid anhydride of a stronger
acid could reduce the acid anhydride of a weaker acid. Unfortunately, procuring phosphorus presents a major obstacle for me :-), and I don't think I
quite have the wherewithall to construct jsc's apparatus! For one thing, I don't think my backyard is big enough :-)
George A Bell
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Managed to make a small amount of white phosphorus today i added 50 mls of 47 % rust killer to 25 grams of crushed heat bead, and heated the mix up
until it was like black lumpy honey ,then put it in a s/s retort i made at work from odd and sods, i then put it in my electric forge and after 3
hours of bubbling and self igniting fumes i was left with 1 gram of white phosphorus ,great but scary stuff .
will try mono ammonium phosphate next ,did a trial run and managed to get a tiny amount of WP so looks promising.
cheers nuxy
    

|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice work. What kind of a flange closure system is that? It almost looks like a Victaulic. Does it require a gasket?
Is the assembly reasonably easy to clean?
[Edited on 12-8-2014 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
There called triclover unions we use them in product pipelines in food and pharmacutical factorys, they can have a nitrile or viton seal and the pipe
to ferrel weld is a purge weld cheers nuxy

|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I knew I'd seen those somewhere before - in a food processing plant I think.
For high temperature work you could likely get a gasket made of graphite. Was your gasket a problem when making P?
[Edited on 13-8-2014 by Magpie]
[Edited on 13-8-2014 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Not really magpie the nitrile gasket go hard after a couple of runs so i use a new gasket work has plenty of them, they smell a bit if the gasket
burns when you open the retort after a run and the phosphorus remnants ignite. cheers nuxy
[Edited on 13-8-2014 by nux vomica]
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Just tried using mono ammonium phosphate mix was 25 gm crushed heat bead 12gm map and 10 gm garden lime hoped lime would convert to ammonium
carbonate and make the phosphate react better, total yield was .4 grm lost some probably .1 grm before weighing ,ph tested retort residue tested acid
on ph paper. cheers nuxy

[Edited on 13-8-2014 by nux vomica]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I am now noticing that you are not using any SiO2 or boria and therefore likely not forming any insoluble residue. This would nicely solve the
problem of how to clean your expensive apparatus. If you can get your yields up I think you are onto something here. 
Edit: Out of curiosity I calculated the %yields for your two experiments:
1st experiment. Assumptions: charge is 50ml of 47% H3PO4 and 25g of carbon. P content = 30.6g. %yield = (1g/30.6g)x100% = 10.3%
2nd experiment. Assumption: charge is 12g H2NH4PO4, 25g of carbon, and 10g of Ca(OH)2. P content = 3.2g. %yield = (0.5g/3.2g)x100% = 15.5%
Do these results look right?
[Edited on 13-8-2014 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Magpie  |
1st experiment. Assumptions: charge is 50ml of 47% H3PO4 and 25g of carbon. P content = 30.6g. %yield = (1g/30.6g)x100% = 10.3%
|
Correction to above post:
1st experiment. Assumptions: charge is 50ml of 47% H3PO4 and 25g of carbon. P content = 9.7g. %yield = (1g/9.7g)x100% = 10.3%
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Will have to delay any more tests as the element in my forge has burnt out, anyone have any ideas on a more efficient condensing arrangement as i was
getting lots of smoke from the phosphorus being carried through the s/s pipe and hitting the atmosphere instead of condensing, i see in a old book i
have that the tubes dip under the surface of the water a fair bit, would the increase of pressure that it gives make the phosphorus condense easier,
it says the water is hot so i don't think its a cooling effect, maybe i should have more expansion space so the vapors slow down enough to condense
before they hit the water. cheers nuxy
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Got the new element off ebay fitted and have changed the retort setup to try and use gravity to make the phosphorus flow out easier,
Used 30 gp heat bead mix that had been used for another batch but that batch was run until exhausted of phosphorus so there shouldn't be any map
residue left in it but the lime mix was still in it so don't no how much there actually was in it, 15 grams of map in granules like sugar mixed
together with spoon then welded the retort shut and turned on forge.
Run took 45 min to get first sign of phosphorus then rest was out in another 50 min got a yield of 2.25 grams works out to a 56.25 %yield would be more one big blob caut on fire on surface of water when i let the water get to
hot and the end of the retort tube must of had some left where i couldnt get it with a wire scraper cos i had a nice fire burning out of the tube
after i had shut down cheers nuxy works out to a 56.25 %yield would be more one big blob caut on fire on surface of water when i let the water get to
hot and the end of the retort tube must of had some left where i couldnt get it with a wire scraper cos i had a nice fire burning out of the tube
after i had shut down cheers nuxy
     
[Edited on 31-8-2014 by nux vomica]
[Edited on 31-8-2014 by nux vomica]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That's a great yield - the highest I've seen. Phosphorus is hard earned as it resists mightily its separation from oxygen. That piece of phosphorus
sitting on your scale looks angry.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
I knocked together a second retort today so i can run several batches in the one day, never bothered to have my own tig at home as i can always use my
work tig on my own jobs at lunch time but i have to wait till I'm back at work to refill the retort now i have gone away from the triclover fittings.
Filled one retort with 15grm map and 30 grm heat bead mix, and the second one with 15 grm map and 30 grm heat bead mix plus 5 grms of garden lime
interesting to see if there is differencece with the lime to no lime Other photo is a page from old book from 1920,s that has some info on phosphorus
production . cheers nuxy
 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
So, if I understand correctly, you are welding the retort pot shut then charging it with powder through the tube, right, or maybe charging then
welding? If so, then how do you open the retort for cleaning and reuse? Do you have to cut off the retort floor, so to speak?
When heating the retort do you apply any heat to the tube or is conduction sufficient? How long is that tube and what is the diameter/height of the
pot?
What's the purpose of the nut welded to the pot?
I can't read that old text. Could you try again with more light and perhaps a better focus.
It's nice to see someone experimenting again. But be careful, phosphorus is a backstabing bitch.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Here's the text in a clearer format, Magpie.
"Phosphoric acid is separated from insoluble calcium sulphate by filtration. it is then mixed with finely powdered coke or charcoal, concentrated to a
thick paste, and heated to bright redness in clay retorts, A, placed in a furnace. Phosphorus distills over, and the vapour is condensed under warm
water in D. The liquid obtained is allowed to solidy (in tubes?)"
"A" refers to the four retorts in the center of the furnace, "D" to the two containers of water on either side. Essentially, this described the
originally posted process, with no addition of silicon dioxide. I think I may try this soon, my furnace will just need a couple of adjustments. That
and I have to make a badass retort like nux's.
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Magpie the main part of the retort is 50mm x 1.5 wall tube that is 50mm long the small tube is 12mm x 1.5 wall and is about 300mm long the nut is a
standoff to raise the bottom of the retort off the firebrick.
I fill the retort first then weld the outlet end on if you look at my previous post there is a s/s mesh disk inside the retort to stop the contents
from blocking the outlet.
If you want i can photograph more pages and not be so lazy and get them in focus.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks No Tears. I find it interesting that no silica or boria is being used. That way you can avoid the slag that is such a pain for cleaning.
This may facilitate P gas escape also. The slag might also plug your screen.
I not sure what the addition of garden lime, Ca(OH)2, would do. I guess you will find out.
It would be nice if you could find a more simple closure system than welding: maybe a bolted flange with a graphite gasket, or maybe a screw cap
with graphite gasket.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
I find that the phosphorus was condenseing around the triclover fitting and not flowing out, a smaller tube hopefully makes the gasses flow through
quicker and wont form a heat sink effect,
I thought that the lime would form ammonium carbonate but i see the heat makes the ammonia volitize out leaveing H3PO4 in the retort (took better
photos of pages  ). ).
cheers nuxy
 
|
|
|
| Pages:
1
..
45
46
47
48
49
..
60 |