| Pages:
1
..
50
51
52
53
54
..
60 |
dactyl
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Red phosphorus from dilute nitric acid and copper (I) phosphide
According to this journal, it might be possible to make red phosphorus from the addition of copper phosphide to dilute nitric acid; details are not
given into this as the study's objective is somewhat different. Nevertheless, a precipitate of red phosphorus seemed to appear upon mere addition of
the copper phosphide
Attachment: ja01450a002.pdf (472kB)
This file has been downloaded 564 times
|
|
|
BluePlanet1
Harmless

Posts: 41
Registered: 30-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemetix  | | Great post! Refractory is critical to so many processes, your rediscovery is certainly going to help me design some high temp work.
|
Awesome, thanks!
Quote: Originally posted by TheNerdyFarmer  | What sort of clay do you use?
Edit: Also what is your youtube channel? I'd like to check it out.
[Edited on 7-2-2017 by TheNerdyFarmer] |
The clay I bought was a cheap, ShopRite brand kitty litter. It cost $4 for 25lbs worth. And I couldn't find an MSDS or ingredients (I checked
everywhere online) but I'm pretty sure it's normal bentonite clay. It's gray colored & looks and acts just like bentonite.
All I do is pour a bunch in my blender and it shatters to a fine dust immediately. You can also add hot water directly to the coarse pellets, stir it
real well then mold and cook. And the coarse version will cook real hard like a brick.
The downsides with the coarse version are:
1) You really need to stir, pound and mold with a lot of muscle. At least 5-10 mins for small test batches and 30+ mins for larger batches.
2) The bricks come out coarser with more micro-cracks.
You'll save a lot of time blending first since it clays up instantly and can be molded and fired within minutes. The bricks come out smoother and even
when I quickfire I barely see any cracks. If you fire it slow and mix with magnesium oxide it makes great crucibles.
AFA my youtube channel I'll get a link up soon. Right now I'm just cooking and storing as many chems as possible to test on video:
1) calcium hydroxide (calcium chloride + lye)
2) calcium oxide (cooked hydroxide)
3) magnesium carbonate (washing soda + epsom salts)
4) magnesium hydroxide (milk of magnesia)
5) magnesium oxide (cooked hydroxide or carbonate)
6) anhydrous silica 99% pure ("crystal kitty litter", the clear pellets)
7) aluminum silicate clay (clay kitty litter)
8) sodium silicate (lye + silica)
9) calcium silicate (lime + silica, doesn't react properly)
10) magnesium silicate (Mg(OH)2 won't react with silica at all, no idea why)
11) perlite
On camera I'll remake certain blends I've been testing cause I BELIEVED that calcium silicate would be far superior to sodium silicate and that
magnesium silicate would be far superior to calcium silicate as a binder.
The problem is Ca(OH)2 & Mg(OH)2 are so insoluble in water that they don't react with silica. If you mix them dry with medium heat (1100c) they
still don't react.
Then at 2500C I thought I'd get porcelain and again, nothing happens. It just melts together then pops and breaks right back to a dust.
Normal portland cement is suppose to be made with lime + sand... but if you mix pure hydrated lime with 99% pure silica... the stuff just sits... it
will appear to "bind"... until the next day when it dries it loses all its strength.
Then sodium silicate like I said before, it binds really well with silica or perlite at room temp and gets hard like a rock. But the second you bring
fire into the equation it expands, cracks, and loses it's properties as a "binder".
Bentonite clay is suppose to be mostly aluminum silicates (phyllosilicates).
And the clay acts like you'd expect magnesium silicates to act. They're not highly soluble in water like sodium silicate. But they bind well with
water somewhat like sodium silicate. And it has a couple unique advantages. Rather than expanding 10xs it size like waterglass... it shrinks about
10%.
So it's minimal shrinkage & very stable. Especially when mixed with 10-25% perlite you barely notice any shrinkage at all. And the main advantage
it actually withstands high heat without spalling and cracking to garbage. Then the more you fire it the harder and more ceramic-like it gets.
In the longrun all my furnaces will be built with clay. But I still wanna document and debunk a lot of bad advice I see on YT.
So I'll do like 5 videos just on refractory. Maybe 10 short videos on white phosphorus like a small series. A couple click baity videos with
armstrongs mixture (chlorate + WP) and a mini WP bomb. Then maybe I'll dedicate a YT channel to high temperature chemistry.
That's something I've always wanted to do is make a really cool but unique chem channel. Chemistry is inherently so boring for most people but I love
it, I have some experience with marketing & audio editing I just need to learn to write and edit the best videos possible. 
ps. To any mods reading this I know I'm driving you nuts I do apologize. Future posts I'll keep as videos on WP, refractory for WP... & anything
off topic I'll post on reddit or other relevant SM threads if they exist.
[Edited on 12-2-2017 by BluePlanet1]
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
You should definitely start a channel on high temp chemistry. There are very few videos on that.
|
|
|
BluePlanet1
Harmless

Posts: 41
Registered: 30-1-2016
Member Is Offline
Mood: No Mood
|
|
Thanks I definitely will.
I just got done testing 5 baby forges & made a huge discovery.
That saying "one small step for man, one giant step for mankind"... that's how I feel about this right now.
Anyone who read my first post (this year) about commercial firebricks will understand the value of this. I talked about how fumed silica is used to
make the best firebrick in the world.
Well I just discovered a way to make something that's NOT technically "fumed silica", but it is:
A) super easy for anyone to make
B) looks identical to fumed silica
C) behaves just like fumed silica (moves with static electricity)
D) produces a firebrick & end product that mimics both the strength & durability of commercial grade firebrick
I'm not saying it can withstand 2 million PSI like industrial firebrick. I'm not saying I'd use this stuff in a commercial blast furnace.
But to make a better firebrick you would need either A) bag of real, fumed silica or B) hydraulic press or C) both.
For now on I'm calling it "faux fumed silica". And I'll post pics if anyone wants to see but the video will show how incredible this stuff is. It's so
light if you rub a balloon on your head then put it by the sides of the bucket it follows the balloon up the sides. And the bricks are smooth as
cement peanut butter. They get tiny needle size pores after cooking but you won't see a hairline crack anywhere.
Will update soon! 
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Silica isn't really fire brick. You want aluminum oxide. Good refractories are 90+% alumina. The lower percentage is usually mullite bonded with the
remainder being silica. Phosphate bonded can be 99% alumina and exceed 3000F.
[Edited on 18-2-2017 by macckone]
|
|
|
BluePlanet1
Harmless

Posts: 41
Registered: 30-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by macckone  | Silica isn't really fire brick. You want aluminum oxide. Good refractories are 90+% alumina. The lower percentage is usually mullite bonded with the
remainder being silica. Phosphate bonded can be 99% alumina and exceed 3000F.
[Edited on 18-2-2017 by macckone] |
I hear you. Many people forget that silicone melts lower than iron and pure silica will melt in a propane forge.
But people also forget that silica combines with alumina to make alumina silicate which is the clay I'm using. And magnesium oxide melts 800C higher
than alumina.
Also, the recipe I'm using with silica was inspired by an old firebrick factory. If you watch between 27:00 - 27:55 How It's Made - Fire Brick that's why I'm using silica. Anyone interested in making WP should watch that whole video. When you heat silicone
oxides with aluminum silicate clay it does raise the melting point of silica through a complex chain of ceramic like reactions that I can't fully
explain.
--------------
While we're on this topic of (pure) silica's low melting point.... I tried another test last night that's pretty darn interesting.
While tweaking the vent cap on my forge I noticed it's reaching forge weld temps. Then I went back reading the thermal redux with coal. Specifically
the part that says "arc furnace OR gas furnace."
And I thought "why haven't I tried this yet?"
So I mixed up coal with silica and sodium metaphosphate.
I put that mix in a small crucible. I held it at the top of the forge (for better observation of the surface) and noticed that the coal redux DOES
WORK in a propane forge.
The whole mixture goes molten then shrinks like 80% it's size. After I saw some Na silicate with beeds of pure silicone metal. The P4 I let burn off.
Using coal WOULD allow you to add a feedstock. Since the mixture shrinks to almost nothing compared to AL which expands like a mess.
If people go back to page 8 of this thread someone used oxy-acetylene with the Al redux and said it took "3 hours" to finish the reaction. But he also
added COAL which I think set a horrible precedent for anyone considering the coal redux.
A coal redux WILL NOT take that long. Nor do you need oxygen. A simple air-propane forge reduces the mix in minutes. Plus the reaction is safer &
cleaner.
No need to worry about slag clogging your vent. No worrying about explosions. No worrying about P4 burning in the distiller due to the large amount of
CO2 produced. And another huge benefit is no phosphine due to no metallic phosphides.
I just want to make & store 1 more chem (phosphoric acid) cause I also wanna test that with coal down the road. After that I'll get the camera out
and start filming this new forge in action.
[Edited on 19-2-2017 by BluePlanet1]
|
|
|
BluePlanet1
Harmless

Posts: 41
Registered: 30-1-2016
Member Is Offline
Mood: No Mood
|
|
I could really use some help from the forum.
For over a year I've been working to simplify this experiment. With a simple, cheap but highly effective design. Something that regular people can
look at & think "yeh, I get that." So they can go out and easily copy the method with little $ or resources.
And right now I'm at that point.
Today I successfully distilled 2.8gms of very pure WP using the coal redux in a baby air-propane forge that took 20 mins to build.
I put the cold steel retort in the cold forge, this is heavy steel pipe compared to the thin retorts that magpie and roger used. But within 2-3 mins
the whole retort and forge reached a blinding white heat. And seconds later the WP started coming over. Then just as quickly the distiller end failed
while leaking like a sieve.
Aside from the retort this set up is so beautiful because...
1) All materials & components are cheap & easy to buy locally. The hardest material to get is the NaPO3 itself. That's the ONLY thing I really
had to buy online. But you can buy other phosphates at Lowes & use displacement reactions for different salts. The most expensive thing I had to
buy was the $50 TS8000 burner head from Lowes.
2) The overall size of the forge. The walls are less than 2" thick. So the furnace is smaller and more compact than any furnace I've seen in this
thread. Even with a 2" pipe (large batches like rogers) you could easily do that in a small forge 5-6" wide.
3) The fuel is cheap. I have this down where there's just 1/2" of space around the whole retort inside the forge. I screw off the burner on the
TS8000. So the air-propane comes out so fast with so much force it "explode burns" around the retort. It hits that thin space so fast & burns so
efficiently that 95% of the flame disappears in 1-2 mins. The exhaust flames goes from blue to yellow then drops down so low (where the gas is
injected) that you see no flame anywhere in the forge. The whole thing just glows white and howls... but you see no flame which is weird.
4) Because of #3 you can probably cook 1000-2000gms of reagant on 1, $3 tank of propane.
5) You don't need the explosive Al mixture. P4 from coal comes out much whiter, the whole reaction is much smoother and safer to do.
BUT THE BIGGEST PROBLEM IS THE >>>RETORT<<<.
I would suggest than ANYONE thinking of doing this reaction... don't think about the chemistry, don't think about the heat or forge or burner or any
of that stuff. I'll list a million reasons on video why to copy my exact set up.
But I can't give you many reasons to copy my retort or magpies or roger or any other design I've seen in this thread.
Even now that I can weld much better and make these smooth & beautiful welds they don't work cause flux core is garbage. I should've bought a TIG
welder but even if I did that wouldn't make it easier for YOU to copy.
If someone wants to add a HUGE contribution... please figure out a way to make a retort that's refillable, that can be reused, cleaned out quickly and
can also handle 1600C.... preferably with easy to get common materials from name brand stores... and also using technology that's easy to scale big or
small.... that also never leaks.
Because right now I drew up a new schematic for a "compression retort" but not many people are gonna like it.
The design consists of 2 pipes.
A) steel retort pipe (1 1/2")
B) 1/2" steel distiller pipe with 90 degree bend.
To connect the distiller to the retort you need a steel plate (foundation for ramming) with a 1/2" hole drilled through it. The width of the hole has
to be the same width of your distiller.
Then you need a solid metal ramming pipe. This solid pipe also needs a 1/2" hole drilled right in the center.
The way this theoretically works is you put your distiller through the 1/2" hole in the steel base. So your distiller pipe faces up and goes into the
middle of your larger retort pipe. Then you mix up bentonite clay with sand (clay for compression, hard sand for "bite"). And you ram the dry clay
around the distiller similar to how you'd make a rocket nozzle. You slam it 8-10 times with hammer and theoretically the clay will compress hard like
a rock, it'll hold the distiller, it should tolerate extreme heat and since the reaction happens at the base the escaping CO2 and P4 should not react
with the clay.
Then the base will either be capped or rammed. 9/10 of my leaks seem to happen at the distiller since the distiller hangs out of the forge at a cooler
temp then the retort. And also because there's no powder towards the top to act as a 2nd barrier against the P4 gas.
Ramming clay at the top should permanently stop leaks. The downside is I can't pack the mixture towards the bottom like I normally do. Which might
just reverse the leak. But if I need I might pack everything like a solid rocket. Ram the distiller in. Pack the reagant in towards the top. Then ram
a clay base in.
And maybe just drill out the base after every reaction I'll have to see.
My main point would be if anyone plans on copying me you should go become an expert on compression fitting steel. Compression gaskets normally use
rubber. But it may be possible to somehow use gaskets with screws... where you remove the rubber gasket and pack it with dry clay... then when you
tighten the bolt that squeezes 2 pieces of steel together which sandwich the clay creating a high temp seal.
I have no idea but we'll see if the clay by itself works....
[Edited on 21-2-2017 by BluePlanet1]
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
If I'm understanding your question correctly, why not use a rubber compression fitting? Weld(or braze) steel plates to the retort and
distiller ends, and bolt them together with an o-ring in between them? Use an o-ring, perhaps, that is a couple of inches larger in diameter than the
tube(s) (to keep the rubber away from the hot gasses), and water cool the joint, either with spray, or a water jacket, or submersion, etc. I'm
assuming the whole length of the tube doesn't need to be white-hot. If you're using mild steel, this material has a much lower thermal conductivity
than, say, copper, so it's possible to have a temperature gradient from one end to the other. Stainless steel would be even better in this regard.
How is the retort holding up to air oxidation? At the temperatures you're using, I imagine that the combustion is possibly occurring catalytically on
the surface of the steel.
As an aside, steel is going to melt below 1600°C; I don't think it's getting that hot. If it's white-hot, it's going to be too bright to look at,
and it's going to light up the area like a quartz halogen lamp. Do you mean maybe bright yellow, maybe around 1000°C? If you have a means of
measuring it, that would be useful information.
[Edited on 2-21-2017 by WGTR]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I like the fresh approach of getting away from the explosive Al/NaPO3 reaction. Now you will be using chemistry closer to that of industry. As you
say this allows you to just concentrate on the engineering problems associated with high temperature. I presume the furnace interior will operate at
1000°-1200°C, ie, white heat or very near it.
What are your design criteria? I will propose some for a start. Please modify to what you have in mind:
1. Re-useable equipment. Retort/condenser good for at least 20 batches.
2. Affordable. say <$200, including the furnace.
3. Batch yield of at least 15g P.
4. P vapor leakage rate acceptable for outdoor or fume hood use, say <1g.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
BluePlanet1
Harmless

Posts: 41
Registered: 30-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WGTR  | | If I'm understanding your question correctly, why not use a rubber compression fitting? Weld(or braze) steel plates to the retort and
distiller ends, and bolt them together with an o-ring in between them? Use an o-ring, perhaps, that is a couple of inches larger in diameter than the
tube(s) (to keep the rubber away from the hot gasses), and water cool the joint, either with spray, or a water jacket, or submersion, etc. I'm
assuming the whole length of the tube doesn't need to be white-hot. If you're using mild steel, this material has a much lower thermal conductivity
than, say, copper, so it's possible to have a temperature gradient from one end to the other. Stainless steel would be even better in this regard.
|
That's a great idea. I have a HUGE blindspot in the respect I'm not measuring the temp where the distiller starts. And I was just at Lowes but their
infrared guns only go up to 317C. The boiling point of WP is said to be 280C.
That's really not hot at all and some heat proof rubber seal should be able to handle that heat. Only issue is the infrared gun gives me a very tight
range of 37C. But I like this idea a lot. If I simply manipulated & monitored temperature better I could get away with things I never thought were
possible. 
Quote: Originally posted by WGTR  | | How is the retort holding up to air oxidation? At the temperatures you're using, I imagine that the combustion is possibly occurring catalytically on
the surface of the steel. |
Yep, I think that's it. A film forms around the whole retort after every time I run it then chips off like a thin layer of oxide paper.
It's hard to see but my smaller retort is coated with a black layer of oxide that chips off if I peel it:

Quote: Originally posted by WGTR  | As an aside, steel is going to melt below 1600°C; I don't think it's getting that hot. If it's white-hot, it's going to be too bright to look at,
and it's going to light up the area like a quartz halogen lamp. Do you mean maybe bright yellow, maybe around 1000°C? If you have a means of
measuring it, that would be useful information.
[Edited on 2-21-2017 by WGTR] |
I've been wondering that myself. The reason I said 1600C is when I put the crucible in with the silica/coal/NaPO3 mix I clearly saw the whole
mixture turn to a molten state. I was shaking the crucible and saw bubbles (CO2) as the coal bubbled up & down and within a few minutes the
whole mixture shrunk down like 80% its size.
When I saw that I thought "damn, that's melting the silica". Which should be hot.
But reading more I may have just witnessed the intermediate reaction where the NaO bonds with the SiO2.... I should know this off my head but it's
something like NaO.SiO2.PO4 that's created as the intermediate and then the coal pulls off the oxygen and when it finished I just saw a little bit of
silicate, small, metallic silicone beeds & some green impurities.
It's definitely getting hotter than 1100C because copper melts almost immediately and I also forge welded 2 steel rods (mild steel?) so thats where my
estimate of 1500-1600C came from.
As far as the color I'd say it's around 2/3 - 3/4 white with 1/3 - 1/4 yellow. So it does have a yellow tinge. The steel doesn't get true blinding
white like my oxyhydro torch but I do see flash spots (in my eyes) if I look at the bottom then look away. With HHO if I look too long I get flash
spots for a good 5-10 minutes. That was before I got my goggles. With the forge I see them maybe 10-20 seconds then it goes away.
Quote: Originally posted by Magpie  | I like the fresh approach of getting away from the explosive Al/NaPO3 reaction. Now you will be using chemistry closer to that of industry. As you
say this allows you to just concentrate on the engineering problems associated with high temperature. I presume the furnace interior will operate at
1000°-1200°C, ie, white heat or very near it.
What are your design criteria? I will propose some for a start. Please modify to what you have in mind:
1. Re-useable equipment. Retort/condenser good for at least 20 batches.
2. Affordable. say <$200, including the furnace.
3. Batch yield of at least 15g P.
4. P vapor leakage rate acceptable for outdoor or fume hood use, say <1g. |
Magpie! Nice to speak with you again. 
I like what you're doing there with design criteria. 20 batches is fine if the final retort can be thrown together relatively quick.
The furnace can be built right now for $70. $20 for the clay and silica (2 big bags) then $50 for the torch.
A batch yield of 15 gms would be acceptable for my larger retort.
Then yields of 2-3 gms are fine for my smaller, testing retort.
I really need 2 retorts that function in a similar way. And I already have 2 furnaces 1 small that can handle 25-40gm charges then a bigger one that
can cook 300-500 gms. That's just based on failed prototypes for the retorts I've been using.
-------------------
On a side note I want to show people exactly what I'm looking for. I bought this today just for "inspiration" and this is EXACTLY the design I'm
looking for, it's the perfect size, perfect thickness, everything is perfect EXCEPT it's made of freakn copper. 
IF ONLY Lowes made these things out of steel it would be so easy to buy one (the copper one was $6) connect any metal tube,
tighten it with an o-ring and get to work. Then after it's cooked I can use a long rotating wirebrush with my drill to clean the inside.
zoom out:

zoom in:

The problem is they have nothing close to that made out of steel. I've gone through all their steel pipe. The closest pipe to that is just fence post.
But it's too wide and the metal is too thick... it would be nightmare to forge something like that into that shape.
I'm never giving up though. I need a good infrared gun. And I'm gonna stare at this copper retort for another day before I go back to Lowes and figure
out something. I could use the copper for Al but I really need steel, coal is the way to go the engineering is just a nightmare. =/
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Would this help?
https://www.google.com.au/search?q=co2+canister&safe=str...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I think it would be useful to prepare a design for a complete retort/condenser assembly. This would be an unrestricted design, i.e., not worrying
about cost. Material selection would be optimum and metal machining would be acceptable. Cooling jackets and heaters would be available to get the
optimum temperature at each location as the P vapor liquified on its way to a receiver under water. Inert gas at pressure would be available.
Features for cleaning would be optimum. The design would include all dimensions.
Of course this would be too expensive to actually build. Its purpose is a starting point from which one could simplify to an affordable design.
My thinking is that instead of starting with what is cheaply available we should start with the optimum and work down to the affordable. Optimum
fitup to your furnace would seem to be a logical starting point.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Brilliant minds think alike, Magpie. This is a very solvable problem if your approach is taken.
I'm currently obligated to finish my project converting ammonia to nitrates, but if I had more time, I'd make a small aluminum vacuum chamber with
acrylic windows on top and bottom. A small piece of firebrick could support a small steel crucible, and a coil of Kanthal could be wound around the
crucible, supported by bits of firebrick. A precooled target of aluminum, with enough thermal mass to handle the crucible charge, could be suspended
over the crucible opening to condense the phosphorus. The reaction would happen under vacuum, and could be done on a lab bench. Upon cooling, the
chamber could be flooded with cooled water that had been degassed by boiling, and the phosphorus scraped off the target underwater.
[Edited on 2-23-2017 by WGTR]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
BluePlanet1: please show us a picture of your furnace. Does the vapor pipe exit the furnace vertically or horizontally?
What are the internal dimensions of the working volume?
I'm working on a retort/condenser design.
Edit: How difficult is it to remove the slag from the retort? Can it be easily knocked off or scrubbed off with a wire brush?
[Edited on 24-2-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
BluePlanet1
Harmless

Posts: 41
Registered: 30-1-2016
Member Is Offline
Mood: No Mood
|
|
Sorry folks! I was crowded with work and still am so I'll respond to anything I miss tomorrow.
Just so everyone knows the second I saw j-sum's post last week my eyes lits up & I immediately went on ebay to order:
2 - 12gm CO2 cartridges
2 - 25gm CO2 cartridges
2 - 45gm CO2 cartridges
2 - 90gm CO2 cartridges
The first 4 arrived today. And the 12gm are way too small but the 25gm are about the same size as my small retort. I must say I'm very intrigued about
using these as retorts. They look like stainless steel (I have to peel the labels off). The bodies are very sturdy like mini scuba tanks. And what's
neat is the copper tube I bought threads over the necks by hand. If I just buy steel tube the same size and o-rings I see no way how these could leak.
The major & only issue I see are the necks. The body on the 25gm is 4"L X 1"W. And the necks are exactly 1cm wide.
But still... we have 4 bigger retorts coming with bigger necks AND the coal slag isn't really a "slag" at all.
It's hard to explain & I'll take pics tomorrow but the "slag" I got was composed of perfectly round silicone beads of various different sizes.
Then what adheres those beads to the steel is a very brittle, very pure looking layer of sodium silicate. That layer of silicate UNLIKE the Al mixture
is very brittle. The problem is those silicone beads. I got about 3-4 big beads the size of BB pellets (that I left in my crucible to test) and if
those beads form too big inside the retort that would be my main objective... how do I get metallic silicone beads out of a steel retort?
From a quick read on wiki silicone metal is highly unreactive. Though I saw one line that says "reacts with weak alkalis" so I'm gonna test that right
now with lye.
------
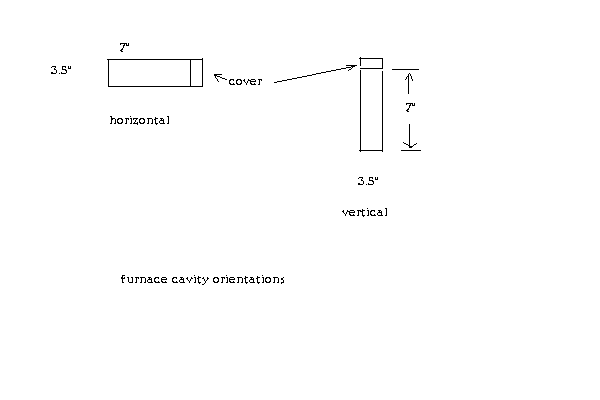
And real quick for Magpie. I'll get pics up tomorrow but the vapor pipe exits the furnace horizontally and bends down vertical. The furnace itself
stands horizontal with 4 bolts I used as legs.
The internal dimensions are exactly 7" deep X 3 1/2" wide. Made out of a 1 gallon stainless steel paintcan that is 7 1/2" long by 6 1/2" wide. This is
the exact can I used: https://www.lowes.com/pd/Valspar-1-Gallon-Residential-Paint-...
My smaller furnace is 4" long X 1 1/2" wide (inside).
The outside is 5" long X 3" wide.
And yes the thicker, braided wire brush pops the beads off although a flat screw driver works faster. For the small cartridges the mini screw drivers
are too short so I'll just use a long, thin rod and maybe bang the end flat.
My goals right now are:
1) See if lye water can dissolve (or just shrink) silicone beads.
2) Buy o-rings tomorrow.
3) START FILMING & FIRING THE COAL REDUX!!!!
Then the bigger retorts will arrive with bigger necks and this actually might work brilliantly. Though I don't wanna deter anyone from submitting
their own retort designs. 
I'll update tomorrow & cheers for all the help.
ps. I need to thank j-sum again. I just checked the size of the 45 & 90gm retorts I bought (with a chart online) and those bigger retorts are
gonna work perfectly. The necks are around double the size I see no way how they can clog, fail or leak. The outside of the steel will slowly oxidize
but I can probably get A TON of testing done with these... I'm so happy right now.
[Edited on 28-2-2017 by BluePlanet1]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for the data, BP1. I can now complete my retort/condenser design.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
First a disclaimer. I have not read all 52 pages of this thread.
The whole P making thing can be summed up, ( IMO, it's a bit like chlorate making).
It's all about the retort (anode).
Since welding seems to be a problem and iron appears to do the job OK (I presume?) would the following work. Easy to clean out. Fairly easy to make.
Just some cutting and drilling and buying.
A steel pipe a few inches in dia, two flat plates with an output pipe and some bolts
I assume steel will do the job.
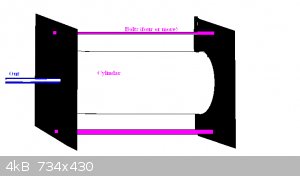
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
BP1:
I'm having trouble understanding the structure of your furnace and how it accommodates the retort/condenser.
I understand the furnace cavity is cylindrical, 7" high by 3.5" in diameter. Now, is this cylinder horizontal (laying on its side) or vertical? If
horizontal is the cover on the side?
Now if this cylinder is vertical is the cover (lid) on the top?
I hope this sketch better explains my questions:
[Edited on 1-3-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Sorry for such a shoddy diagram and explanation.
I am simply repeating and agreeing with BluePlanet1 above that it's all about the RETORT.
The diagram is a diagram of a retort. No furnace shown.
A pipe (cylindar of iron), two end plates with bolts sandwitching the pipe (you could weld one end plate on if you like) and a smaller outpipe pipe
coming from the retort (wherever it is convient).
I am assuming steel is up to the job, is that a correct statement? or that steel will last a few runs.
The retort is easy to make and easy to clean out and can be made fairly large.
I intent to try this sometime.
As the opening poster (The Polverone  ) said himself: ) said himself:
Such a common atom and so hard to get you hands on it (or something like that).
[Edited on 1-3-2017 by yobbo II]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
My retort/condenser consists of 3 parts, ie, a barrel, an end cap, and a condenser. The first two parts are shown below. I will submit a sketch for
the condenser soon. The internal end of the black iron end cap is to be lapped flat. A 2mm graphoil ring gasket will be used to seal the cap to the
barrel.
https://www.industrialgaskets.com.au/uploaded/GRAPHITE%20SHE...
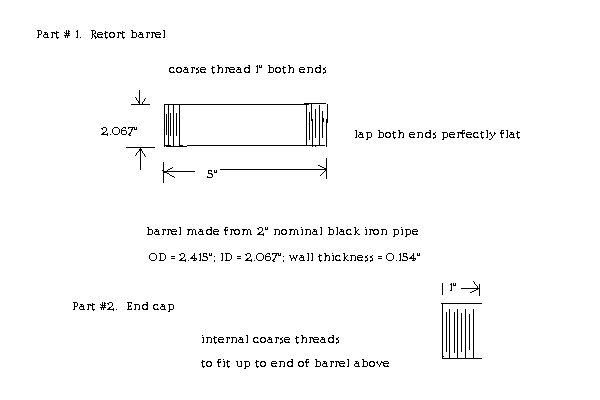
[Edited on 1-3-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
One problem with screwed on endcaps is that you may not be able to unscrew them (or at least one of them) to clean out the retort after each run.
Will they not be seized up?
The graphite gasket is only good to 870C.
With the bolts (my retort above) you can simple cut the bolts.
If you can weld there will be no need for bolts or screwed on end caps. You can simply cut the pipe in a ring (tidy) clean out, recharge and weld
again.
[Edited on 1-3-2017 by yobbo II]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, my design depends on there being no leakage at the end caps. Graphoil is likely not the best gasket material. Pure graphite may be better.
It would be nice to be able to make your own gaskets out of asbestos sheet, but I don't think it is available anymore. Ceramic fiber gaskets such as
those available from this vendor may be adequate as they claim they are good to 2300°F (1260°C):
http://www.mercergasket.com/ceramic_fiber_gaskets.htm
----------------------------------------
edit: This page in the above reference claims a graphite gasket can be good to 5000°F (2760°C) in a reducing environment. Hot P vapor would
certainly be a reducing environment:
http://www.mercergasket.com/materials_guide.htm#Carbon%20and...
--------------
Your design depends on a metal-to-metal seal which mine also gives if a gasket is not used.
[Edited on 1-3-2017 by Magpie]
[Edited on 1-3-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Various silicone RTVs are available that work at engine
temperatures for exhaust manifolds, they turn rock hard
above the 'working temp' of (700F for permatex ultra
copper).
The gasket material for exhaust manifolds is usually
just metal sheet with a carbon face material that will
handle even higher temperatures. Copper is commonly
used as a gasket material for high heat applications.
There is also less effective fiberglass rope gasket material
which should be available from any wood stove vendor.
The fiberglass rope gasket often comes with a silicone
that handles high heat. Again it becomes rock hard glass
above the working temp.
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
There would be very little pressure in the system. The bottom gasket (assuming you are standing up you 'cylindar retort') is not really needed as the
stuff in the retort would seal the bottom. Some fire cement or something like that would do around the top.
The only pressure in the system would be the pressure needed to drive the gases out of the end of the output pipe which you have under water at its
end. If the pipe end is not much under the water then there is little or no pressure in the retort.
If you had a flange on the top end of the retort (welding needed so not so simple for some) then there would be more area to form the seal.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I agree that there should be very little pressure in the system. But a leak of P vapor in the furnace is a very ugly and dangerous thing - it
happened to me. Billowing white fumes of P2O5, an intensely hot fire, nothing you can do but let it burn out. Granted I was using a very thin walled
paint can. Add in the fact that 2 of our forum members (Rogermeryaw and Endimion17 were badly burned by P. These events foster a certain amount of
conservatism in my thinking.
[Edited on 2-3-2017 by Magpie]
[Edited on 2-3-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
| Pages:
1
..
50
51
52
53
54
..
60 |