| Pages:
1
..
57
58
59
60 |
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
Great news! A 2019 paper (attached) published in the International Journal of Materials and Metallurgical Engineering describes the process of
producing phosphorus from the carbothermic reduction of phosphoric acid. This is strong evidence that the method I am trying can work.
Rather than heating the acid gradually in the tube as the Japanese researchers did, I am planning to pump the acid in with a peristaltic pump once the
carbon is already hot. The procedure will be as follows:
1) Placing both ends of the peristaltic pump's tube into a beaker of phosphoric acid, purge the peristaltic line of all air by pumping the acid in a
loop out and back into the beaker
2) Flush system (furnace, phosphorus collection flask, schlenk line, ...) with argon
3) While keeping peristaltic line and furnace connection tubing submerged in the acid, use tubing joiner to connect peristaltic line to furnace
connection tubing (the tubing can be lifted out of the acid once the tubes are joined and a seal is made)
See the attached picture for a visual.
Attachment: Carbothermic-Reduction-of-Phosphoric-Acid-Extracted-from-Dephosphorization-Slags-to-Produce-Yellow-Phosphorus-.pdf (431kB)
This file has been downloaded 709 times 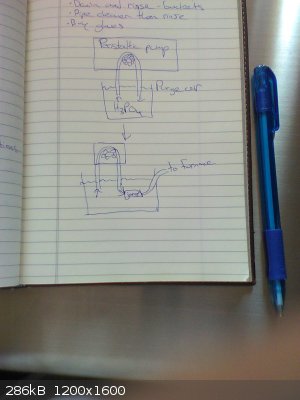
[Edited on 1-7-2020 by Duff]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
I cant find any iron retorts online x( gonna have to learn metalworking at the makerspace, or buy a custom part.
Does anyone know where to buy a metal retort, or how I can make one from stuff you can get at home depot? Cant use glass for this.
[Edited on 7-4-2020 by Cou]
|
|
|
metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
I succeeded.
I heated 7g (NaPO3)6 + 3g Al + 2g silica sand in a steel tube retort with a copper tube leading into a jar with water. I heated it to 700 C, after 15
minutes I did not see anything, I removed the retort, the bum of the retort was 900 C (measured with my infrared pyrometer). Then I saw 'water'
dripping from the retort tube, but it catched fire with a small bright yellow flame smoking with dense P2O5 smoke. So it were drops of liquid P4 which
spilled. Fortunately the amounts were less than a gram and my fumehood is completely fire resistant and I was aware of the dangers of P4, but a
WARNING applies !
When you distill P4, be prepared that P4 might spill and keep bare hands, clothes or anything AWAY and use leather gloves and a face shield and
perform outdoors or in a well ventilated fire resistant fumehood !
My experiment went well, but when it goes wrong it goes BADLY wrong !
P4 on your skin results in nasty and painful burns !
[Edited on 2020-7-15 by metalresearcher]

|
|
|
garphield
Hazard to Self
 
Posts: 58
Registered: 9-12-2019
Member Is Offline
|
|
Not sure if this would work, and due to using metal phosphides it would be pretty dangerous even if it did, but would it be possible to reduce a
phosphate salt with aluminium in a thermite-style reaction, then oxidize the aluminium phosphide in the slag to AlCl3 and PCl3/PCl5 via putting the
ground-up slag in an inert solvent and bubbling chlorine gas through? If you need elemental phosphorus you could probably use zinc powder or something
to reduce the phosphorus chlorides to zinc chloride + phosphorus. No idea if this would work tho.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by metalresearcher  | I succeeded.
I heated 7g (NaPO3)6 + 3g Al + 2g silica sand in a steel tube retort with a copper tube leading into a jar with water. I heated it to 700 C, after 15
minutes I did not see anything, I removed the retort, the bum of the retort was 900 C (measured with my infrared pyrometer). Then I saw 'water'
dripping from the retort tube, but it catched fire with a small bright yellow flame smoking with dense P2O5 smoke. So it were drops of liquid P4 which
spilled. Fortunately the amounts were less than a gram and my fumehood is completely fire resistant and I was aware of the dangers of P4, but a
WARNING applies ! |
Very nice!
The success with silica makes me wonder if other acidic oxides might liberate P2O5 from NaPO3. V2O5 is the most acidic solid oxide I can think of that
will withstand the high temps.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Just a thought, but reacting sodium hypophosphite with an oxidizer should make elemental phosphorus. Its a byproduct in electroless nickel plating: https://en.wikipedia.org/wiki/Electroless_nickel-phosphorus_...
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
If you mix hypophosphite with oxidizer you get phosphite or phosphate.
Phosphorus which forms in nickel plating is in form nickel phosphorus alloy, it doesn't form as a pure element.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Then dissolve away the nickel with an acid, that might work
|
|
|
B(a)P
International Hazard
    
Posts: 1116
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
This is an interesting concept.
Not that many will have the ability to perform electrolysis on a liquid at 850 C.....
https://pubs.acs.org/doi/pdf/10.1021/acssuschemeng.0c04796
Attachment: yang2020.pdf (3.8MB)
This file has been downloaded 612 times
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
I decided to just buy red phosphorus from chemcraft.su. I need it for regioselective bromination of alcohols. I don't do any illegal drugs so I'm not
worried about the possibility of police coming knocking. If they search my house expecting to find empty Sudafed boxes, they're wasting their time.
My life will not be complete until I can regiospecifically brominate alcohols. Civil asset forfeiture a risk I'm willing to take.
[Edited on 11-6-2020 by Cou]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by Cou  | I cant find any iron retorts online x( gonna have to learn metalworking at the makerspace, or buy a custom part.
Does anyone know where to buy a metal retort, or how I can make one from stuff you can get at home depot? Cant use glass for this.
[Edited on 7-4-2020 by Cou] |
I accidentally found these examples:
https://indonesian.alibaba.com/product-detail/high-recovery-...
https://indonesian.alibaba.com/product-detail/mercury-retort...
It seams that this type of retorts is used for distilling of mercury from gold amalgam (so, search "mercury retort". I don't know whether this type of
retorts is suitable for producing of white phosphorus because they have a very narrow pipe.
[Edited on 6-11-2020 by teodor]
[Edited on 6-11-2020 by teodor]
|
|
|
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
Thought I'd give an update. The amount of time I've been able to spend on chemistry has been limited over that past few months, but hopefully I can
spend more time on phosphorus production in the coming months. Right now I'm thinking hard about how I can effective do "unit testing" of various
parts of my setup, e.g. can the o-rings on my flange valves withstand the required pressure and temperature, can my condensers sufficiently cool the
gases exiting the furnace, dealing with possible phosphine formation, etc.
Another problem I've been wrestling with is how to place the activated carbon in the furnace's quartz tube in a way that ensures any phosphorus
produced will not condense before it leaves the tube. The tube is 30 cm long, but the heating zone in the furnace is 10 cm long. I may have to place
the tube so that the exit flange is closer to the heating zone.
There might be an easy solution to the problem of phosphine formation. The attached document claims that
| Quote: |
Sodium hypochlorite in aqueous solutions reacts practically instantaneously with phosphine so that such solutions are particularly suitable for
removing traces of phosphine from a gas stream |
So passing the gases through a solution of sodium hypochlorite at some point might be an idea.
I want to minimize the amount of combustible or toxic gas that enters my pump, so if I can remove phosphine from the gas stream before it gets there
that would be fantastic.
There are so many hypotheticals, I think at some point I just have to admit that I can't account for everything, and just go ahead and try to do it,
if something doesn't work out I can try to understand why and try again. I just haven't had time to work on this for a while.
Attachment: 2494-The-Chemistry-of-Phosphine807f.pdf (857kB)
This file has been downloaded 424 times
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
Have you produced any phosphorus yet?
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. We talked about this a decade ago. It seems that no-one followed through.
"When triphenyl phosphate was reduced with a solution of LiAIH 4 in carbitol, which was prepared as described in [9], the formation of red phosphorus
was observed and not even traces of phosphine were present."
LiAlH4 or NaAlH4, are not easy "gets"... But, Phosphorus has become much harder. Metal Hydrides can still be purchased. Phosphorus and Phosphorus
Chlorides, have become highly restricted.
So... Back to the original paper, wherein some guys were failing to produce the desired Phosphine, and they were instead producing useless "Red
Phosphorus"
https://www.sciencemadness.org/whisper/files.php?pid=191992&...
[Edited on 17-3-2021 by zed]
[Edited on 17-3-2021 by zed]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Interesting rxn. P4 production with glassware and normal temperatures.gamechanger. weird solvent though.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
zed, that's a great find. But I'm wondering: how do you produce triphenyl phosphate? Maybe you can vacuum distill a mixture of metaphosphoric acid and
phenol at equilibrium?
PhOH + (HPO3)6 >> 4 H3PO4 + (PhO)3PO (g)
Presumably the formation of triphenyl phosphate is disfavored but the lack of H-bonding may imply some higher vapor pressure? bp 244 C at 10 mm Hg.
I've never heard of esterifying phosphoric acid directly, though, but this patent implies that the big problem is olefin beta-elimination and that
might be avoided with phenol:
https://patents.google.com/patent/US4921990A/en
But phenol itself is already more volatile than triphenyl phosphate...
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Thats an insanely expensive reaction to run for something as mundane as red p.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Red Phosphorus is insanely hard to acquire in the USA. Guys that want it, may have to scrape it off of matchbox strikers. Yellow, AKA White
Phosphorus, is hard to come by also, but it is more "makable".
Completely converting White to Red, isn't totally easy.
Initially, the restrictions had to do with Domestic illegal Methamphetamine Manufacture. Now, it is restricted six ways from Sunday. Chemical
Weapons Ban, etc... There are restrictions on international commerce, as pointed out in that Japanese Procedure at the top of the page. Nations that
don't normally produce their own P, are concerned.
And yet, for many reactions, chemists must have it. In the USA, Colleges and Universities, are often doing without. Too much paperwork. No problem
for large corporations, I suppose. But, for the amateur, damned hard to come by. I'm gonna go back and swipe a quote from that paper.
"Due to this huge energy consumption, current yellow phosphorus producing countries are limited to China, the United States, Kazakhstan, and Vietnam.
After the United State banned the export yellow phosphorus as a strategic commodity from 1996, other yellow phosphorus producing countries also
limited their export. Thus, Japan's imports of phosphorus ore, as well as yellow phosphorus, are becoming increasingly severe year by year."
White Phosphorus isn't especially hard to make, but the process requires a committed approach. Brutal perhaps. A backyard, An old BBQ, wood or
charcoal, and a source of forced air, are required.
Argon forced through your reaction mixture might improve yields by conveying Gaseous Phosphorus out of the reaction mix. Most mixes work something
like this: Mix up Phosphate, Carbon, and Sand, then heat it up hotter than the hinges of Hell. The Carbon reduces the Phosphate to P, then exits as
CO2. The Silica or Sand... Helps. As I said, additional Gas flow might help the P to get to where it needs to go.
Here, Rogeryermaw, shows you how it is done. https://www.youtube.com/watch?v=mibM4WUx74Q&t=2s
https://www.youtube.com/watch?v=4YjUC2dmNV0
https://www.youtube.com/watch?v=23bXKpLyXJk
A further procedure by Canadian Chemist
Also brutal.... https://www.youtube.com/watch?v=NxQrqXIMEh8
[Edited on 21-3-2021 by zed]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Going back to something that was said earlier, https://pubs.acs.org/doi/pdf/10.1021/acssuschemeng.0c04796. Possibly using a deep euctectic mixture of Calcium chloride and urea/choline chloride
spiked with calcium phosphate would be electrolyzed to give phosphorus? The temp doesn't seem to play a part, and if we can keep it around 100C or so,
then it solves the problem of molten salt electrolysis.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Link doesn't work for me
Reflux condenser?? I barely know her!
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Wouldn't electrolysing calcium chloride and a phosphate give off some pcl3 ?
Or does the phosphorus form at the opposite electrode than the chlorine?
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
The paper is called: A New Concept for Producing White Phosphorus: Electrolysis of Dissolved Phosphate in Molten Chloride
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You need to ensure that phosphorus is reduced before any other component of the solution. That isn't going to be easy. Urea will likely react before
phosphate does, or it will react with the byproduct. You also need not only to melt CaCl2 but to dissolve Ca3(PO4)2 in it.
Now, you might be able to just electrolyse molten sodium metaphosphate, which melts at 628 C. That already gets you a bit lower. But it's extremely
corrosive. Electrolysis in CaCl2 has received intense attention lately due to its role in the refinement of Ti and Zr.
The eutectic of NaCl and CaCl2 has a composition of about 49:51 [NaCl]:[CaCl2] or about 43 grams NaCl to 85 grams CaCl2, and melts around 500 C:
http://www.energy-proceedings.org/wp-content/uploads/2020/03...
So you might start there. If you're lucky, you could run the process at around 550 C instead of 850 C, which puts you in the operating range of more
ordinary materials, but still not Pyrex.
The MgCl2-NaCl-CaCl2 eutectic melts at 420 C which is still just a bit too high for glassware, and anhydrous MgCl2 is difficult to prepare, but that
gets you a bit lower. The MgCl2-NaCl-KCl eutectic (55:24.5:20.5 wt%) is around 387 C:
https://www.osti.gov/servlets/purl/1440736
Whether a useful amount of phosphate can be dissolved in the chloride salts and electrolysed at these low temperatures is not at all guaranteed, but I
suppose you can always try. However, glassware is still not recommended because phosphates will corrode it. Chlorides attack steel.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
If I understand the paper, only 2 percent of the molten salt solution is phosphate by weight so even if this is feasible for the amateur, at a small
scale it seems quite pointless.
Reflux condenser?? I barely know her!
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
But possibly topping up the phosphate periodically will solve that problem. And maybe a euctic that won't be reduced first would work. I think I'm
going to try this some time in the next few months.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
| Pages:
1
..
57
58
59
60 |