| Pages:
1
2 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
trends in chemistry education
We have all heard about the pressures on organic chemistry teaching labs to cut costs, reduce risk, and reduce waste. I mentioned before that in my
travels I like to check out undergraduate organic chemistry lab manuals at nearby universities to see what experiments are being done nowadays. The
last time I did this the manual showed virtually no syntheses.
There is always some mention of virtual experiments where the whole "experiment" is done on a computer. 
Here's another variation where the instructor does the experiment then the student only writes it up:
https://www.youtube.com/watch?v=r9XtV-KxV2o
I'm glad now is not my time as a student in the teaching labs. I look back fondly at my experience in "the golden age" of organic teaching labs where
almost every experiment was a synthesis in my own hands.
I know that this disturbing trend is not true for all colleges and universities. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Unfortunately the Accountants are in control.
Planned costs like wages, equipment, reagents, waste disposal are frowned on, yet acceptable.
Un-planned costs like damage claims screw up their figures, which annoys them a lot.
Sadly the current population Sues for whatever they can get on the slightest excuse.
For these reasons Teaching actual practical applications of Chemistry (and other practical disciplines) are declining, which follows through into a
declining manufacturing capability.
Best place to teach and learn ? Probably China.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Don't forget that the few syntheses that are still done in the lab are now almost universally microscale. Who knows what's going to happen when
someone needs to actually prepare a useful quantity of anything?
I have used the smallest glass I have ever seen. There are combination still heads and receivers, and the largest flask is something like 10mL. It's
all in 14/10. This counts as a distillation:
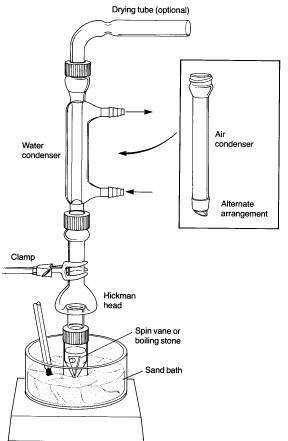
That's a 3ml vial as the boiling flask.
I can see the utility of this apparatus in a teaching environment, but the danger here is that students leave a chem course without understanding that
on larger scales, many of these reactions would run away. They will not know what to do when a runaway happens on a larger scale, and they aren't
taught about keeping a bucket of sand around in the event of a fire. They do not know the danger of liter-sized flasks imploding under vacuum. They
will not know how to properly grease a joint, nor will they have experience selecting condensers, columns, clamp support, stirring methods, and
heating systems appropriate for larger applications.
Take a look at this lab, especially the list at the end:
https://www.siena.edu/assets/files/general/Microscale_Solubl...
[Edited on 19-1-2016 by Praxichys]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It saddens me that Knowledge will be lost in this way.
I do not Teach as i do not have the patience or empathy for it.
(teaching things unrelated to Chemistry, obviously).
For those that Do teach, it must be excruciating to be so restricted.
If there is an Upside, waste products and disposal thereof are considered First, which is good.
Edit:
The List at the end, if even considered by early Chemists, could simply not exist, as no discovery would ever have been made.
[Edited on 19-1-2016 by aga]
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
this trend is by no means a recent phenomenon. I finished teaching 20 years ago and even then I was constantly being berated for exceeding the safety
boundaries in a school setting. cant let the little darlings get bicarb on their precious fingers or anything; and the production of dimethylmercury
was definitely out 
Beginning construction of periodic table display
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
whilst we did a lot of small scale synthesis in my first year, most of it was not that extreme! we got to use tens of mmol scale which certainly
enough to feel exothermic nature of some of the reactions (jones oxidation of menthol). I'm surprised we even were allowed to use jones reagent
(although pre-prepared for us) when most people didn't even know what a RBF was. I do agree with doug though, we did soxhlet extractions with 1 gram
plant material (to extract coumarin maybe) which resulted in a coloured piece of filter paper which is what we were marked on (wtf). I rarely tell
anyone i have a home lab at uni because the first thing they ask is if i make m3th. This is obviously very disappointing as I would expect at an
academic facility people would not be such imbeciles and think like that.
|
|
|
TheAlchemistPirate
Hazard to Others
  
Posts: 151
Registered: 25-3-2014
Location: The point of no return
Member Is Offline
Mood: Enigmatic
|
|
I'm not too disappointed with my high school chemistry class labs, probably because I didn't expect much  . I only joined to understand the things I was already doing at home. Unfortunately I can never tell anyone about most
of my experiments that I'm excited about as they are a little on the fringes of chemistry. So, when my hands are dyed deep yellow from making picric
acid (I tried wearing gloves to stop it.) , I just try to keep them out of sight and don't mention anything. In my first semester, there
were two reactions in which the balanced equation was actually known to the class. The rest (about six) were just simple demonstrations of a color or
heat change without an explanation of the chemicals involved. . I only joined to understand the things I was already doing at home. Unfortunately I can never tell anyone about most
of my experiments that I'm excited about as they are a little on the fringes of chemistry. So, when my hands are dyed deep yellow from making picric
acid (I tried wearing gloves to stop it.) , I just try to keep them out of sight and don't mention anything. In my first semester, there
were two reactions in which the balanced equation was actually known to the class. The rest (about six) were just simple demonstrations of a color or
heat change without an explanation of the chemicals involved.
Interestingly our teacher liked to avoid mentioning the names of chemicals which had a "bad" reputation in those unnamed reactions. In one example,
they were showing endothermic reactions. We were given a bag to fill with water, and a mysterious white powder to place around the it, inside a
ziplock. When the bag was popped, the ziplock became very cold. Of course this mysterious powder was prilled ammonium nitrate, and this was basically
a cold pack. I made a point of asking the teacher a question regarding the reaction, referring to the powder as ammonium nitrate. I was met with
strange looks  . There was a similar incident with iodine IIRC. . There was a similar incident with iodine IIRC.
To the credit of the school, however, I'm unsure of if I would trust my classmates with highly chemicals or distillations either  . Seriously, though, what is the point of a "virtual experiment" ?! How can you
observe a chemical phenomenon scientifically if it is already designed by someone else? I don't know how any of my classmates keep any sort of
interest without performing any actual chemistry themselves, which none do as far as I know. . Seriously, though, what is the point of a "virtual experiment" ?! How can you
observe a chemical phenomenon scientifically if it is already designed by someone else? I don't know how any of my classmates keep any sort of
interest without performing any actual chemistry themselves, which none do as far as I know.
"Is this even science anymore?!"
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
I don't know how to describe this but chemistry class is dead. Two or so labs a year, in an honors class, and a curriculum that cripples itself to
reach further. Despite dealing theoretically with complex molecules and theories, about half the class can't identify something as simple as Manganese
Dioxide from its formula without using a periodic table. Our chem teacher notices this, and is dying inside.
I think most of this comes from a lack of interest in the profession from the student body, instead an obsession for marks. No students give an actual
shit about Chem. Its hard to explain but this makes the class so cringeworthy that I should just take a video for the SM members. If the students
don't care, why should the teachers help them? 9/10 questions in class arent the 'what' or 'why'. Its mostly "will this be on the exam?" Cumon I mean
you should be in this class to learn, not just for the marks.
[Edited on 20-1-2016 by Hawkguy]
|
|
|
kt5000
Hazard to Others
  
Posts: 133
Registered: 27-3-2013
Location: Southwest US
Member Is Offline
Mood: Final exams
|
|
I read an article in Science magazine a couple months back quoting a western chemist teaching at a Chinese university.. He said the university was
receiving 100% approval on grant applications.
|
|
|
Scr0t
Hazard to Others
  
Posts: 118
Registered: 14-1-2012
Location: Europe
Member Is Offline
Mood: Desiccated
|
|
Quote: Originally posted by aga  | Unfortunately the Accountants are in control.
Best place to teach and learn ? Probably China. |
That reminds me of one Chinese student I remember who came over to my former University who was quite bummed out because his father would not let him
study chemistry as it was "too dangerous" and instead insisted that he take business studies instead.
Poor kid!
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Surprisingly (to me, at least) those microscale set-ups that are universal in academia now are extremely hard to get on the open market.
I have one such kit, but spare parts for it (septa, gaskets, replacement glass) are unavailable - the vendors won't even talk to individuals.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Yeas, they're common in what I've seen in the countless letters I get from small colleges asking you to go to them (it's that time in my life...).
SIUE, for whom I know a professor of chemistry at, does research with that sort of micro-scale stuff. RESEARCH. They have large glassware, but it is
never used. The main research there is done on usage of more intuitive drug development, where heterocyclic chemistry isn't needed as much, and
'building blocks' are used instead.
But the glassware thing is sad. I hope OSU, the local college I'll probably go to, uses normal size stuff...
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quote: Originally posted by careysub  | | Surprisingly (to me, at least) those microscale set-ups that are universal in academia now are extremely hard to get on the open market.
|
That surprised me too. I tried to buy one about a year back and couldn't find anything, not even from China.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Is there anyone here on this site who had an account with someone like Ace who could do orders for microscale stuff?
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
When I was in high school about ten years ago we did experiments with potassium dichromate. These experiments on oxidation states were done without
hoods or gloves, only goggles. Each pair of students had a bottle with around 100g of solid K2Cr2O7 to use as well as
other chemicals. I forget the exact experiment but it still involved handling what today would likely be regarded as an extremely hazardous
material...
Thinking back... Maybe our school was a bit too lax though 
Note to self: Tare the damned flask.
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
Quote: Originally posted by Magpie  | We have all heard about the pressures on organic chemistry teaching labs to cut costs, reduce risk, and reduce waste. I mentioned before that in my
travels I like to check out undergraduate organic chemistry lab manuals at nearby universities to see what experiments are being done nowadays. The
last time I did this the manual showed virtually no syntheses.
There is always some mention of virtual experiments where the whole "experiment" is done on a computer. 
Here's another variation where the instructor does the experiment then the student only writes it up:
https://www.youtube.com/watch?v=r9XtV-KxV2o
I'm glad now is not my time as a student in the teaching labs. I look back fondly at my experience in "the golden age" of organic teaching labs where
almost every experiment was a synthesis in my own hands.
I know that this disturbing trend is not true for all colleges and universities. 
|
This is how my online high school physics classes and my chemistry class worked. Though the chemistry course used real (pre-bottled dilute solutions
of
) chemicals, I'm pretty sure the current ones do not. I was disappointed by the change.
[Edited on 1-21-2016 by Diablo]
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
youtube
Quote: Originally posted by Mailinmypocket  | When I was in high school about ten years ago we did experiments with potassium dichromate. These experiments on oxidation states were done without
hoods or gloves, only goggles. Each pair of students had a bottle with around 100g of solid K2Cr2O7 to use as well as
other chemicals. I forget the exact experiment but it still involved handling what today would likely be regarded as an extremely hazardous
material...
Thinking back... Maybe our school was a bit too lax though 
|
I liked NurdRage's video on that.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I'm suprised, in talking with my chemistry teacher about experiments I've done, safety doesn't come up too much. Every so often there's questions of
wether I did something outside in a nonconfined area, but little worries as to the reagents I use. A nice change from my former honors chem teacher
who was alwaysworried about my use of acids, strong bases etc. But they both seemed to care and have interest in the results of the projects I had,
which is nice. Sorry about any spelling errors.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I don't remember much concern about safety in chemistry in college or high school except when working with diethyl ether or mercury, but usually the
quantities of reagents were pretty small. On the other hand, I do remember things like seeing fireballs produced by people heating carbon disulfide
over an open flame and puddles of mercury in the sink.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Haha, that's pretty bad. Though that definitely doesn't sound like modern chemistry classes.
We of course use very small scales, though I suppose that's a cost thing too.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Actually, in terms of reagents used, I am not sure there is a lot of cost saving in using micro quantities. My experience is that there tends to be a
lot of chemicals held by schools that are stored for years and deteriorate rather than being used up.
For a school, chemicals are a much smaller part of the budget than the capital expense of building a lab and outfitting it. It is also a lot smaller
than the staffing and maintenance costs.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by j_sum1  | | Actually, in terms of reagents used, I am not sure there is a lot of cost saving in using micro quantities. My experience is that there tends to be a
lot of chemicals held by schools that are stored for years and deteriorate rather than being used up. |
I think some chemicals are bought for a specific project or research. Then when that project is done the chemicals are not needed anymore and can sit
on the shelf for many years. Also if a particular experiment is removed from the curriculum then the required chemicals can become excess.
Quote: Originally posted by j_sum1  |
For a school, chemicals are a much smaller part of the budget than the capital expense of building a lab and outfitting it. It is also a lot smaller
than the staffing and maintenance costs. |
My local college experimented with microscale one year then went back to small scale using 19/22 glassware. This was before my time,
probably about 15 years ago.
I've wondered how big an expense waste disposal is for the teaching labs in organic chemistry. My school did eliminate an experiment using
demercuration as it generated a drop of mercury.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by j_sum1  | Actually, in terms of reagents used, I am not sure there is a lot of cost saving in using micro quantities. My experience is that there tends to be a
lot of chemicals held by schools that are stored for years and deteriorate rather than being used up.
For a school, chemicals are a much smaller part of the budget than the capital expense of building a lab and outfitting it. It is also a lot smaller
than the staffing and maintenance costs. |
That is definitely true. See here for the story of how I was able to rescue a lot of old sodium iodide from my school. I neglected to mention there that I also rescued a
few hundred grams of lithium fluoride which was in mint condition, but very old. Another problem at my school is that sometimes a teacher will keep a
container of a chemical in their classroom without returning it to the stockroom, so then another teacher will buy a new container when they need it
but can't find it. Thus we have many nearly full open containers of commonly used reagents, which results in even more things being thrown out.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by j_sum1  | Actually, in terms of reagents used, I am not sure there is a lot of cost saving in using micro quantities. My experience is that there tends to be a
lot of chemicals held by schools that are stored for years and deteriorate rather than being used up.
For a school, chemicals are a much smaller part of the budget than the capital expense of building a lab and outfitting it. It is also a lot smaller
than the staffing and maintenance costs. |
Pike and Mayo (I think) cite the advantage that microchemistry makes it feasible from a cost perspective to use some very costly reagents (I think
they cited some rare metal reaction, and of course there are many scarce biological molecules).
I have no idea to what extent that is possible advantage is realized in practice.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
19/22 is just cute, but I certainly would prefer it to microscale. Too bad the glassware laws in Texas weren't sized based, at the very least.
|
|
|
| Pages:
1
2 |