| Pages:
1
2 |
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Pyrimethamine Synthesis?
Ok so Im sure everyone has heard or read about the scandle involving Daraprim and how the CEO of a pharmaceutical company (not sure which one) mr
Martin Shkreli raised the price of the drug from $13.50 a pill to $750 a pill making it even more expensive than cocaine. Now this happened back in
September so Im sure the price has gone back down to something reasonable but I decided to do some quick research into the drug and found that its
actually a medicine used to treat or prevent certain protozoan infections. It is used i believe as an anti malaria pill as well but how it treats
people with immune deficiency disorders like HIV is that it helps to treat a parasite called Toxoplasma gondii which infects humans with a condition
called Toxoplasmosis. Toxoplasma gondii is that weird little parasite that has a life cycle between cat and mice that works by making the mice more
attracted to cats so then the mouse is more likely to be eaten by a cat and so allowing the life cycle to continue. Toxoplasmosis is also well known
to affect humans in a normally non lethal way and maybe even affect there habits somewhat making people more attracted to cats and resulting in
something i like to call crazy cat lady syndrome, of coarse this might not actually be true and is subject to more research but if you have an immune
deficiency disorder it may cause the parasite to become lethal and cause potential problems.
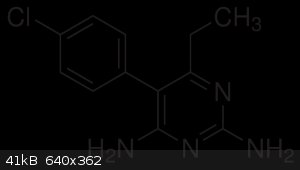
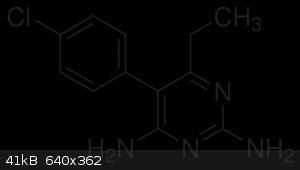
Basically i want to synthesize this compound, not because i live in a tropical country with malaria but because i really fucken want to and it might
be fun although it might be a wile before i am able to do the experiments.
Ive done a bit of research into how i would go about synthesizing this compound and have thought about starting from aspartic acid and this other
compound that i am having alot of trouble naming but i thought it was a phosphic acid methyl glycine or something similar to acetamide which you can
see in this first image number 2.

From what i understand the ATCase is an enzyme that uses phosphoric acid as a leaving group but i want to do a similar reaction only using a different
leaving group in an amination to yield Carbamoyl aspartic acid which could then be dehydrated into 4,5-dihydroorotic acid, im not sure what leaving
group would work in this fashion and i will need a bit of help on the reaction.
Next my idea was to carry out a Arndt-Eistert reaction using ethanediazonium which is kinda scary because its an azide. I was wondering if the
Arndt-Eistert reaction had to used specifically a chloride or could it be substituted with another halide and if so can phosphorous tribromide be
prepared using white P instead of red P?
I would then follow that up with a decarboxylation to yield 4-ethyl-2,6-dioxo-1,3-diazinane or 4,5-dihydroorotic acid with an ethyl group instead of a
carboxylic acid.
(sorry cant find a picture)
From 4-ethyl-2,6-dioxo-1,3-diazinane i would carry out a reductive amination although i dont want to use AlHg for this because theres 2 ketones to
reduce and this i think will reduce my yields to something stupid, so i have some platinum and i was wondering if there is a way to carry out a
hydrogenation using Adams catalyst (since thats easy to prepare) at standard pressure without using a pipe bomb apparatus?
Then im not sure if it would be a better idea to carry out an oxidation of the pyrimidine ring using a platinum catalyst to reform the double bonds
before halogenating the 5 position or to do the oxidation after.
The next step would be the halogenation of the 5 position on the pyrimidine structure but im not sure what halogenating agent would do that without
attacking the ethyl group on the 6 position.
My simple and perhaps stupid idea was to then perform a grignar reaction with bromobenzene and then chlorinate the para position on the benzene ring
to yield
5-(4-chlorophenyl)-6-ethyl-2,4-pyrimidinediamine.
Now it might be smarter to change the order of some of these reactions to prevent some side reactions but i think ive done pretty good so far, also is
there a better way to attach the benzene ring to the 6 position that doesn't involve using a halide so that i could instead chlorinate the benzene
ring before hand and increase the yield a little bit?
I might have been a little inconsistent with my naming but i think its all correct, i apologize for the lack of pictures but i don't have a cord to
connect my phone to my computer to upload photos of my drawings.
Here is where i got some of my information i cant be bothered doing proper references.
https://en.wikipedia.org/wiki/4,5-Dihydroorotic_acid
https://en.wikipedia.org/wiki/Toxoplasma_gondii
http://www.drugs.com/cdi/pyrimethamine.html
https://en.wikipedia.org/wiki/Arndt%E2%80%93Eistert_reaction
http://www.cdc.gov/parasites/toxoplasmosis/health_profession...
Cheers Assured Fish

[Edited on 1-2-2016 by Assured Fish]
|
|
|
Texium
|
Thread Pruned
1-2-2016 at 06:57 |
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
There are much easier ways (and I also see numerous problems with your scheme).
Consider starting from (4-chlorophenyl)acetonitrile, as in this paper: http://pubs.acs.org/doi/abs/10.1021/ja01152a060
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
To me, the funny thing about the pyrimethamine is, according to the synthetic scheme on wikipedia, aside from the starting material
(4-chlorophenyl)acetonitrile, the precursors are relatively simple and mandane. No, that's not the funny thing, that's the setup to the punchline. I
phrased it wrong. The funny thing is that unsubstituted phenylacetonitrile is a recognized precursor to amfetamine: there are probably a number of
'meth labs' with stock of this compound right now.
If the 4 chlorination works well, or if the 4-dechloro pyrimethamine is effective (which is not necessarily likely! some times, that one little change
makes a terribly big difference) then there is potential for a hilarious situation where charitable 'meth cooks' can contribute to the public good.
Not saying it's likely, it would just be amusing.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Halogenation of an unsubstituted phenyl ring is quite hard, at least without destroying the rest of the molecule .. Also I'm pretty sure the not
chlorinated version of this drug is not going to be effective in any way.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | then there is potential for a hilarious situation where charitable 'meth cooks' can contribute to the public good. Not saying it's likely, it would
just be amusing.
|
You would probably not be able to accomplish this before someone else fixes the price issue, you know? Even if you could, you can't distribute this,
and I would recommend against using it.
In any case, the requisite 4-chlorophenylacetonitrile might be obtained by an Ullmann-Goldberg-type reaction between p-dichlorobenzene (mothball) and
acetonitrile with CuI and a strong base.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
The drug is available from many compounding pharmacies with a prescription, the FDA has said that it will allow that due to the issues surrounding the
current drug source. The active ingredient is available cheap from two companies to pharmacies. So eventually people will find cheaper sources.
TCI sells it for $113/5g.
I do hope that Shkreli ends up in jail, as he is a greedy bastard. But the price will come down eventually, once some other generic suppliers get
through the FDA process. Shkreli tried to make that difficult, but they can't stop other companies for but so long. If Turing goes bankrupt, then
other companies will be much less likely to try such crap. And if enough people write their congressperson, then they can fix the mess they made in
the laws to allow such crap. Shkreli is no more a scientist trying to cure disease than Bernie Madoff.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
This scandal is no more odious than than the story of Harvoni. The ingredients were developed to a certain extent with the aid of U.S. taxpayer
money. After several buyouts, the material is now controlled by the Giliad corporation.
Harvoni has become the method of choice for treating Hep C.. If you can afford it. Other wise, you can eventually die, unlamented.
It runs about $1000 dollars per tablet in the U.S.. Over 100,000 dollars for the 12 week course.
The tablets can be manufactured for about a dollar each. And, the initial investments have already been recovered. No relief in sight. At least in
the U.S., where the company has spent freely on lobbying efforts.
Trust me, in most of the rest of the world, the price is much, much, lower. Especially so in India. The Indian government saw the need for the drug,
and the scale of the price raping the original manufacturers were engaging in, and they flat out refused to issue an Indian patent. No patent,
equals, no proprietary rights....equals the product entering the public domain. A free for all.
In India, the course of treatment, costs about a thousand dollars. Period.
The inventor has a right to make money. Lots of it. But, at a certain point, a reasonable government will exercise the right of eminent domain.
Sorry! Too important to the public health. We are buying you out.
[Edited on 2-2-2016 by zed]
[Edited on 2-2-2016 by zed]
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Zed,
The pricing strategy in pharma , as you probably know, has little to do with cost of product development and production. These costs, despite what we
are told, are quite low overall. The price is set by "value to the patient/customer" as determined by marketing studies. That often means how much
will you, the patient, pay to not die. Of course much of the ultimate cost is born by insurance, ie. the rest of us. Consequently, at least in the US,
the patient never is really faced with the real bill for treatment. That is why in large part drug costs in the US are so high.
AvB
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Harmoni is somewhat different. There are many other treatments available, most of which are not as good or effective, but people don't die overnight
of Hep C, it is mostly harmless until it creates chronic problems. But delaying treatment for a while has little effect. And the drug will come off
patent eventually and be much cheaper. But Gilead did spend a lot to to the clinical trials, which now cost millions to billions of dollars, so they
do deserve some ability to recoup their investments, but I will agree that they are charging too much, but as new treatments come out (and they are
coming quickly), the price will fall quickly, which is why they are in such a hurry to make something before the drug becomes obsolete. And little
if any government money was used for the R & D on it. So that is the way that things should work, only the prices have become too high now.
Daraprim was discovered by Burroughs Wellcome (by Nobel prize winners, Elions and Hitching), a drug discovery group owned by a charitable trust, many
years ago, and was sold at nearly cost for many years. When BW started becoming financially untenable, their board sold the company to Glaxo, who
paid a lot for it, and the new Glaxo-Wellcome was able to continue discovering new drugs, but did raise prices to allow the company to grow better.
The Wellcome trust went from being worth about a billion dollars into a fund worth over 10 billion in a short time, which shows that perhaps
charitable trusts are not good at running pharma companies, as they now fund far more medical R & D than they ever did before. But that is a
whole other story.
The drug went off patent many years ago, but since the market was so small in the US, no generic company was willing to go through the FDA process to
sell it in the US. Eventually GW, (then GSK) sold the marketing rights to Daraprim to another company, which sold it again, each time the new buyer
raised prices. Then Turing bought it, and using a loophole in FDA process, found a way to hold off generics, while also raising the price to levels
way above what it is worth, and he did not do any R & D with that money, only used it to pay off his previous Ponzi scheme victims... So that
case is a combination of no real CURRENT R & D prices, loopholes to prevent generics, and greed. If Daraprim was a newly discovered drug, it
would be allowed some market exclusivity as an orphan drug, but it is not new, so that should not be the case. But the US drug laws have many complex
bits and pieces than Turing is trying to use to create a loophole. That could be fixed easily or the FDA can just allow compounding pharmacies to
sell it, which they have done.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
let us stick to the chemistry rather than debating on what and what not the drug companies should do 
atara is the only one till now to propose a route for the synthesis of the starting compound , p-choroacetonitrile(although there is no reference to
back it up)
It would be good if someone found out a route to make the nitrile starting from styrene.But sadly,even though I searched a lot,I couldn't find a route
to make p-chlorostyrene directly from styrene 
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I would suggest making 4-chloro-toluene, partially oxidizing that to the aldehyde, making the cyanide out of that and go from there. Long way but
doable without a industrial setup
If you can do the first step on a multi-molar scale the rest is reasonably doable.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
If you have 4-chlorotoluene, you could also try brominating it to 4-chlorobenzyl bromide, and then reacting that with a cyanide salt. This might be a
better way than trying to selectively oxidize to the aldehyde.
However, 4-chlorotoluene isn't available to the amateur chemist (although it is for registered businesses; VWR sells it at $123/kg).
Edit:
One amateur-feasible route to 4-chlorotoluene would be from 4-toluidine by a Sandmeyer reaction.
You could also try directly chlorinating toluene with a Lewis acid catalyst, but you'd have to separate the various products.
[Edited on 2-6-2016 by Cheddite Cheese]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Apologies for not replying sooner
| Quote: |
Cheddite Cheese
posted on 2-2-2016 at 04:30
There are much easier ways (and I also see numerous problems with your scheme).
Consider starting from (4-chlorophenyl)acetonitrile, as in this paper: http://pubs.acs.org/doi/abs/10.1021/ja01152a060
|
This is probably an easier way to go about although i still wanna make some 4,5-dihydroorotic acid which could then be used to make different
pyrimidinediamine analogs.
| Quote: |
Tsjerk
posted on 2-2-2016 at 10:07
Halogenation of an unsubstituted phenyl ring is quite hard, at least without destroying the rest of the molecule .. Also I'm pretty sure the not
chlorinated version of this drug is not going to be effective in any way.
|
Thanks if i follow my route i will oxidize the ring before attempting to attack the 5 position.
I have no intention of synthesizing this drug to sell it or make profit i was just interested in its antimicrobial properties and its synthesis.
I am guess you are all referring to this route.

I dont really know much about the synthesis of guanidine and triethyl orthoformate, but i guess you just throw sodium in methanol to precipitate
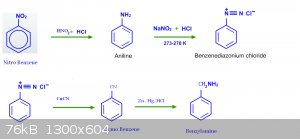
sodium methoxide (sounds like fun) but for the synthesis of (4-chlorophenyl)acetonitrile could you not chlorinate aniline then separate the
o-chloroaniline, then react p-chloroaniline with sodium or potassium nitrate and HCL followed by copper cyanide.
And for the synthesis of 4,5-dihydroorotic acid i was thinking of synthesizing bromocarbamoyl like so
COBr2 + NH3 -----> CH2ONBr
COBr2 being carbonyl bromide
CBr4 + H2SO4 -----> COBr2
Acording to Wiki carbon tetrabromide is used in the plastic and rubber industry but i still wouldnt know where to purchase it ( i live in new zealand)
it could again be synthesized but im not sure how i would go about doing this. Alternatively if i can make phosphorous tribromide from white P then i
could react sodium carbonate with ammonia and then with HCL to precipitate NaCl then react with PBr3.
Could i use Polyphosphoric acid/Pyrophosphoric acid to dehydrate the N-carbamoyl aspartic acid to dihydroorotic acide?
Cheers Assured Fish
[Edited on 6-2-2016 by Assured Fish]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tsjerk  | | I would suggest making 4-chloro-toluene, partially oxidizing that to the aldehyde, making the cyanide out of that |
how would you make the cyanide from the aldehyde ? Quote: Originally posted by Cheddite Cheese  | | If you have 4-chlorotoluene, you could also try brominating it to 4-chlorobenzyl bromide, and then reacting that with a cyanide salt. This might be a
better way than trying to selectively oxidize to the aldehyde. |
but keep in mind that the benzylbromide would be a tear gas and working with cyanide could be dangerous( and also quite hard to get in some places)
if tsjerk can convert the aldehyde to cyanide,then one could easily get the aldehyde from toluene using MASON
Quote: Originally posted by Assured Fish  | | but for the synthesis of (4-chlorophenyl)acetonitrile could you not chlorinate aniline then separate the o-chloroaniline, then react p-chloroaniline
with sodium or potassium nitrate and HCL followed by copper cyanide. |
you would get benzonitrile not phenylacetonitrile
| Quote: | And for the synthesis of 4,5-dihydroorotic acid i was thinking of synthesizing bromocarbamoyl like so
COBr2 + NH3 -----> CH2ONBr
COBr2 being carbonyl bromide
CBr4 + H2SO4 -----> COBr2 |
won't carbonyl bromide have toxicity similar to phosgene ?
[Edited on 6-2-2016 by CuReUS]
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Chlorine is a bit unreactive for these types of things, atara, or not atara... While hydrogen is no substitute for chlorine, perhaps bromine is? In
that case, though it would not be a simple procedure to hit benzene with iron bromide and extra bromine, these are all things that threads on
sciencemadness have dealt with successfully. The reaction with copper acetonitrile is then considerably more likely to work.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
| Quote: |
Quote: Originally posted by CuReUS
you would get benzonitrile not phenylacetonitrile
|
Damn i wasn't paying enough attention. Ok this is tedious and putting in two extra steps and twice the reagents but you could reduce the benzonitrile
to the benzylamine and then again react with copper cyanide and HCL.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, if you could easily obtain p-chloro-phenylalanine, you might be able to deconstruct that molecule to produce your desired starting material.
Or, P-chlorobenzaldehyde is pretty cheap. Reduce it with isopropylalcohol to
P-chloro-benzylalcohol. Convert it to the benzylchloride, and thence to the nitrile.
[Edited on 7-2-2016 by zed]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | Chlorine is a bit unreactive for these types of things, atara, or not atara... While hydrogen is no substitute for chlorine, perhaps bromine is? In
that case, though it would not be a simple procedure to hit benzene with iron bromide and extra bromine, these are all things that threads on
sciencemadness have dealt with successfully. The reaction with copper acetonitrile is then considerably more likely to work. |
I think the best alternative, if you assume you can just buy shit, would be to chlorinate phenol with something mild like NCS, then react with Tf2O,
and use that for the Ullmann reaction. However, I think dichlorobenzene deserves a try -- chlorine is a good EWG, which makes this more reactive than
a monochlorobenzene. Here's a patent for example using p-dichlorobenzene in the Ullmann reaction at 165 C:
http://www.google.com/patents/US4766253
| Quote: | | In U.S. Pat. No. 3,472,782 and U.S. Pat. No. 3,371,120, 1,4-dichlorobenzene is reacted with 3-chlorophenolate at 165° C. under CuCl/KI catalysis to
give 3,4'-dichlorodiphenyl ether. |
It reacts once, but not twice, since the product is less electron-deficient and thus less reactive. And acetonitrilate is probably way more reactive
than m-chlorophenolate (less steric hindrance, much more basic).
EDIT: Attached is a review of the Ullmann condensation with a number of such reactions on aryl chlorides; it appears that carbanions are highly active
in the reaction, so much so that we might be concerned with over-alkylation.
Attachment: rossi2004.pdf (2.7MB)
This file has been downloaded 795 times
EDIT 2: I found a paper discussing mono-alkylation of p-dichlorobenzene specifically, via electrochemistry:
http://pubs.acs.org/doi/abs/10.1021/jo00106a009
[Edited on 7-2-2016 by clearly_not_atara]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Assured Fish  |
Damn i wasn't paying enough attention. Ok this is tedious and putting in two extra steps and twice the reagents but you could reduce the benzonitrile
to the benzylamine and then again react with copper cyanide and HCL. |
I am not sure that would work
Quote: Originally posted by zed  | | Well, if you could easily obtain p-chloro-phenylalanine, you might be able to deconstruct that molecule to produce your desired starting material.
|
I found a reference for making the nitrile with 2-aryl amino acid ,but not phenylalanine ,( they even use p-chloro derivative as one of their substrates to get a good yield of 86% ) ,( they even use p-chloro derivative as one of their substrates to get a good yield of 86% )
https://www.thieme-connect.com/products/ejournals/abstract/1...
| Quote: | Or, P-chlorobenzaldehyde is pretty cheap. Reduce it with isopropylalcohol to
P-chloro-benzylalcohol. Convert it to the benzylchloride, and thence to the nitrile |
an MPV reduction,not bad ,but don't you think it would be easier to do a cross cannizaro with formaldehyde to get the alcohol  .Then IMHO, it would be better to tosylate it rather than convert it into halide for
the CN substitution .Then IMHO, it would be better to tosylate it rather than convert it into halide for
the CN substitution
Quote: Originally posted by clearly_not_atara  |
I think the best alternative, if you assume you can just buy shit, would be to chlorinate phenol with something mild like NCS, then react with Tf2O,
and use that for the Ullmann reaction. |
calm down atara,how will using phenol help us get phenylacetonitrile ? how will you convert the notorious -OH group to CH2CN ?
| Quote: | | EDIT: Attached is a review of the Ullmann condensation with a number of such reactions on aryl chlorides; it appears that carbanions are highly active
in the reaction, so much so that we might be concerned with over-alkylation. |
In this review,there are references for the reactions of aryl halides with cyanomethyl anions and the results are quite bad  (although they are not ulmann reactions ) (although they are not ulmann reactions )
http://pubs.acs.org/doi/abs/10.1021/jo00963a012 (31% yield of acetonitrile with chlorobenzene)
http://pubs.acs.org/doi/abs/10.1021/jo01329a015 (17% yield)
and these reactions are radicle based(like ulmann),so an EWG in the ring would decrease the yield furthur
EDIT: I followed zed's idea and I think I have found an OTC source of p-chloro phenylalanine .Apparantly its a drug called fenclonine
https://raypeatforum.com/community/threads/affordable-source...
you can get p-chloro phenylacetonitrile from that using this reaction
http://www.sciencemadness.org/talk/viewthread.php?tid=32534
[Edited on 8-2-2016 by CuReUS]
[Edited on 8-2-2016 by CuReUS]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Might be better routes, but I have read of Benzaldehydes being reduced to BenzylAlcohols, in Quantative yield by excess Isopropyl Alcohol, under
pressure.
The conversion of Alcohol to Halide is well documented, as is that of Halide to Nitrile.
Involves Na/K CN, which demands care, but it is do-able.
Most of us are familiar with the idea of degradation/decarboxylation of amino-acid to nitrile, but I find no references.......where folks claim to
have reproduced the original experimenters results, with good yields.
P-Chloroaniline has some potential as a starting material. It is cheap.
But perhaps the path is too convoluted. DiazoniumBromide+ Ethylacrylate (anhydrous conditions)=Cl-C6H4-CH2CH(Br)COOEt. Treat with ammonia,
hydrolyse, decarboxylate/dehydrate....To produce the PhenylAcetonitrile.
Oh, nevermind. Now that I have actually written it out, it seems cumbersome. Too many steps, equals too low an overall yield.
[Edited on 13-2-2016 by zed]
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
I like the PDCB route very much, at least, I like the aspect of it that it involves PDCB. Acetonitrile is obtained, if impure, by pyrolysis of
acetamide, which means every component besides time would be readily available to the inhabitants, thus illustrating (though perhaps not
demonstrating, for reasons of health and safety) the value of chemical education. This is probably why Nurdrage has set the compound as his goal,
andhe could be reading the very thread.
If PDCB does not prove to be a good substrate (the latter two papers deal with a radical mechanism, which I didn't think was how the ullmann coupling
works ordinarily, but maybe I'm wrong) for whatever reason, for example, if heat/time is used to force the chlorine, the acetonitrile might be
reprotonated by the product first forming a diphenylacetonitrile; C6H4ICl would probably most simply be made via the grignard reagent
of dichlorobenzene and I2... but PhCl, PhICl2 by decomposition, and maybe terephthalic acid mercury salt (if the "double" hunsdieker reaction works as
well as with benzoate), etc.
A book has suggestions on the topic of aryl iodide installation:
https://books.google.com/books?id=BPcxrmIgLKMC&pg=PA631
Zed's para-chlorophenylalanine would be the most elegant solution.
aka. (thanks, google!) https://en.wikipedia.org/wiki/Fenclonine
"a selective and irreversible inhibitor of tryptophan hydroxylase... It has been used experimentally to treat carcinoid syndrome, but the side
effects, mostly hypersensitivity reactions and psychiatric disturbances, have prevented development for this use. It is used in scientific research in
humans[4] and animals[2] to investigate the effects of serotonin depletion."
I love the internet. You know are ancestors couldn't do anything like this? My love and amazement might seem strange unless you consider that. None of
em. For millions of years. Back the time of the dinosaurs. Even the Flintstones only had television.
[Edited on 17-2-2016 by halogen]
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I've looked at references and there seem to be some issues with using acetonitrile directly, in fact it's very common to see Ullmann reactions
featuring the tetrakis(acetonitrile)copper(I) complex. In Ullmann reactions featuring this complex you never see the alkylation of acetonitrile, and
because the acetonitrile anion is prone to polymerization it might not be very efficient to use bases strong enough to deprotonate it. Most studies
featuring the anion of acetonitrile use it at -78 C or colder and require a superbase like nBuLi. In fact there are essentially no references
to the Ullmann reaction using a carbanion nucleophile to displace an aryl chloride, which suggests that it only occurs with nitro-substituted arenes,
if at all.
However, it does appear that both p-bromochlorobenzene and p-iodochlorobenzene undergo the desired reaction, which is tantalizing. There are other
reactions that feature the alpha-arylation of nitriles with aryl chlorides, such as this:
http://onlinelibrary.wiley.com/doi/10.1002/anie.200351954/fu...
but they all require complex catalysts.
[Edited on 18-2-2016 by clearly_not_atara]
|
|
|
Delta
Harmless

Posts: 11
Registered: 23-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
if tsjerk can convert the aldehyde to cyanide,then one could easily get the aldehyde from toluene using MASON
[Edited on 6-2-2016 by CuReUS] |
Forgive me, but what is MASON. I have heard of partial oxidation of toluene to benzaldehyde using chromyl chloride or chromic anhydride/acetic
anhydride, but not MASON.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | calm down atara,how will using phenol help us get phenylacetonitrile ? how will you convert the notorious -OH group to CH2CN ?
|
In the post I had said "Tf2O" which was a joke, but upon consideration the Appel reaction can be used with triphenylphosphine and sulfuryl chloride
which aren't entirely out of reach, certainly if you plan on doing anything at the scale that would be required to affect the pyrimethamine market you
should probably be able to source TPP. In particular this means that you could convert tyrosine to the nitrile and use the Appel.
https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/fi...
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | I've looked at references and there seem to be some issues with using acetonitrile directly... In fact there are essentially no references to
the Ullmann reaction using a carbanion nucleophile to displace an aryl chloride, which suggests that it only occurs with nitro-substituted arenes, if
at all.
However, it does appear that both p-bromochlorobenzene and p-iodochlorobenzene undergo the desired reaction, which is tantalizing.
|
I am sorry to say atara,but you seem to be talking in riddles.One moment you say alkylation with carbanion is not possible,and the next moment you say
that its possible in dihalogenated arenes.
if there were no references on the alkylation with acetonitrile,how did you know it would work ? was it your own idea ? then you should have mentioned
that .
pls post the references here .
| Quote: | | Most studies featuring the anion of acetonitrile use it at -78 C or colder and require a superbase like nBuLi. |
I don't see why this should be a problem . -78'C is difficult to achieve , but its possible and nBuLi is cheap
Quote: Originally posted by Delta  | Quote: Originally posted by CuReUS  |
if tsjerk can convert the aldehyde to cyanide,then one could easily get the aldehyde from toluene using MASON
[Edited on 6-2-2016 by CuReUS] |
Forgive me, but what is MASON. I have heard of partial oxidation of toluene to benzaldehyde using chromyl chloride or chromic anhydride/acetic
anhydride, but not MASON. |
MASON is an acronym i made for the electrolytic oxidation of toluene to benzaldehyde because I kept on forgetting the name of the salt used.It stands
for Manganese Ammonium perSulphate electrolytic OxidatioN
http://www.sciencemadness.org/talk/viewthread.php?tid=2223&a... Quote: Originally posted by clearly_not_atara  | | Quote: | | calm down atara,how will using phenol help us get phenylacetonitrile ? how will you convert the notorious -OH group to CH2CN ?
|
In the post I had said "Tf2O" which was a joke, but upon consideration the Appel reaction can be used with triphenylphosphine and sulfuryl chloride.
In particular this means that you could convert tyrosine to the nitrile and use the Appel.
|
Hmmm, appel reaction didn't come to my mind but now that you mention it,it sounds like a superb idea . a member recently mentioned the appel reaction
in his post. And the funniest thing is that acetonitrile is used as a solvent in the appel reaction,so we might be able to use the nitrile we get from
tyrosine as the solvent itself   (just kidding) (just kidding)
http://www.sciencemadness.org/talk/viewthread.php?tid=63182#...
the tyrosine route might be the next best thing after fenclonine 
[Edited on 24-2-2016 by CuReUS]
|
|
|
| Pages:
1
2 |