| Pages:
1
2 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Drying Ethanol with Portland Cement
This experiment is to quantitatively determine the drying effect of Portland Cement on azeotropic ethanol over a period of 1 hour.

Portand cement composition :-
(source: http://iti.northwestern.edu/cement/monograph/Monograph3_6.ht...)
CaO 63.8% insoluble in methanol/Et2OH
=> Ca(OH)2 insoluble in EtOH
SiO2 21.5% ?? insoluble in water
Al2O3 4.5% insol. in water. practically insol. in EtOH
Fe2O3 3.0% insol in water. insol in EtOH
MgO 2.4% insol in both
P2O5 0.1% hydrolises. wiki says it is a powerful dessicant
TiO2 0.2% insol in water
Na2O 0.2% =>NaOH Yes, Yes both forms
K2O 0.8% =>KOH Yes, Yes, both
SO3 2.5% => reacts to H2SO4
Many of these components may have already reacted to become different species, given the high temperatures employed in manufacture, however these are
the only accurate data available relating to the composition, and will be used as-is for the time being.
Calculation of the components reactive to water and the stoichimetry, it was determined that 1g of a cement powder will react with a maximum of 0.213g
of water.
With this figure, the minimum weight of cement required to dry x grammes of ethanol with a concentration of c w% was calculated to
be approximately :-
4.7x - 0.047cx
Therefore, to dry 100g of OTC 37.5w% vodka requires at least 293g of cement !
Fractional distillation will generally bring the ethanol to near the 96 w% azeotrope, so the requirement falls to 0.188g of cement per gramme of
azeotrope, giving a more manageable requirement of ~19g cement to dry 100ml.
Objective:-
Plot the concentration of azeotropic EtOH treated with cement powder over a period of one hour or more.
Experiment:-
1. Materials used.
Fractionally distilled ethanol.
Portland Cement powder
Spatula
0.01g scale
Filter papers
80ml disposable plastic beakers
Disposable plastic stirrers
Funnel
Ethanol refractometer
Distilled water
Egg timer
Tin foil, cling film, playing cards, cd-rom cases - anything to cap off the beakers.

2. Make & standardise the stock solution.
Distill a quantity of ethanol using a Vigreux column, yielding at least 100g of azeotropic ethanol.
Dilute 5g of the ethanol with 15g distilled water, then measure with an ethanol refractometer.
Multiply the reading by 4 to arrive at the actual reading.
Repeat 3 times with a clean beaker each time, then average the results.
3. Calibrate the instrument
Calibrate the refractometer to Zero according to the manufacturer's instructions.
4. Sample Preparation
Measure approximately 5g (representing a 33% excess) of cement powder into 7 beakers, marked 0 through 7.
Record the exact weight added to each beaker.
Add a stirring rod to each beaker.
5. Add Ethanol
Fill a clean beaker with approximately 20g of the stock ethanol solution.
Record the weight then add to each beaker, starting with #7.
Mix with the stirring rod.
Discard the rod and immediately cap the beaker and set it aside.
Repeat for all beakers.
When done, start the timer, set for 10 minutes.
6. Prepare to Filter
Add a clean filter paper to the funnel (fluting is not necessary).
Place a clean beaker under the funnel.
7. Filtering
Beginning immediately with beaker 0 (not waiting 10 minutes) tip the entire contents into the filter funnel.
Discard the beaker.
If Not processing beaker 0, reset the timer now.
8. Prepare a sample to measure
Place a clean beaker on the scales, and Tare the scales.
Measure out approximately 2.5g ethanol filtrate and record the weight.
Without re-taring the scales, add enough distilled water to reach approximately 4x the ethanol weight.
Record the total weight of ethanol and water.
9. Measurement
Place a few drops of the ethanol/water mixture onto the aperture of the refractometer.
Wait 30 seconds for temperatures to equalise then record the reading.
Discard the beaker.
Repeat three times from step 8 for each sample.
Working at a resonable rate, there is plenty of time between readings for a quick sip of beer.
10. Process all samples
When the timer expires, repeat from step 6 until all samples are processed.
11. Recalibrate the refractometer
Re-test the calibration of the refractometer as per manufacturer's instructions.
If the result is not Zero, discard the entire experiment and re-do from start.
12. Process results
Adjust each reading to account for the actual weights recorded, then take an average of the 3 adjusted readings for each sample.
Plot on a graph.
13. Work out what the hell it all means.
Results:
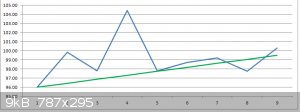
The calculated w% values ranged from 107.84 w% for the stock solution through to 117.29 w% for the maximal Outlier 20 minute sample.
Estimating the stock solution to be around 96 w% a correction factor of 0.89 was applied to the other results, giving a range of 96.0 w% to 100.3 w%
An estimated 'best fit' of the plotted data gives an increasing range from 96.0 w% (stock solution) to 99.5 w% (70 minutes treated sample).
Observations / Concerns:
Given the diversity of the components in cement powder, tests should be done for contaminants introduced, such as Iron and Sulphate ions.
The cement-treated then filtered ethanol was water-clear with no traces of any haze or other colouration, indicating that any contaminants introduced
must be solvated, if present.
Conclusions:
1. Portland cement powder can remove approximately 3 w% water from an ethanol/water azeotropic mix in one hour, representing approximately 75%
efficacy in that time.
2. Despite the (assumed yet scientifically unproven) superior efficacy of K3PO4, CaO, K2CO3 et al, the low
cost and universal availability of Portland Cement renders (sic) it a viable option for amateur chemists to achieve near-anhydrous ethanol, starting
from an azeotropic (96%) mixture.
3. This refractometer and experimenter might not be the sharpest tools in the box for this experiment.
Raw data :
Attachment: Calcs.xlsx (20kB)
This file has been downloaded 350 times
Please deride, slate and comment.
[Edited on 3-2-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Did you check these cement-dried samples with KMnO4?
It's faster than I anticipated.
Plus side: you may now have enough cement to finally build that explosion-proof concrete outhouse? 
[Edited on 3-2-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not yet.
Darkness approached towards the end, and the usual night-time preparations had to be made against the Night Evils.
Tomorrow shall it be so-tested (a new 1 hour treated sample from the same feedstock).
The pot permanganate test is very handy.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Not yet.
Darkness approached towards the end, and the usual night-time preparations had to be made against the Night Evils.
Tomorrow shall it be so-tested (a new 1 hour treated sample from the same feedstock).
The pot permanganate test is very handy. |
A photo, dried v. azeotropic with t'KMnO4, would be of high appreciability, Squire.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
99.5 is pretty good but try it with everclear or 95% EtOH. I'd be impressed if you could get to 99.5.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
The listed composition of portland is a bit misleading, in reality the main components are di- and tricalcium silicate, tricalcium aluminate,
tetracalcium aluminaferrite and hydrated calcium sulphate (gypsum). Some free Ca(OH)2 will also be present, about 1%.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yeah, however that was the best i could find in terms of percentages to work with.
Would you happen to have a more detailed breakdown of cement ?
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
The wikipedia page has some data: https://en.wikipedia.org/wiki/Portland_cement
This has some data on the hydrated species found in cured cement (and CCN abbreviations): https://en.wikipedia.org/wiki/Cement_chemist_notation
It's been a while since I worked with cements (and then mainly physical testing) but I do remember some. I have to add that I listed the
clinker composition, most cements also contain blast furnace slag and/or fly ash. Both of these are rich in calcium and aluminum
silicates.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
from vague memory, Portland cement kilns are sometimes used for toxic waste disposal,
what isn't destroyed by high temperature will be trapped in the concrete.
So chemical purity of the alcohol as a reagent may be variable,
and human consumption would be unwise.
Could you use Plaster of Paris for a similar effect?
EDIT: I just looked at the Wikipedia entry for Portland cement pointed to in the post above, it mentions waste disposal there.
[Edited on 4-2-2016 by Sulaiman]
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I'm pretty rusty on the hydration mechanisms, but I believe the free CaO is the key. It dissolves, lowering the pH so the other ingredients can
dissolve and form gels that eventually hardens as the water is consumed. So other than trace amounts I don't think you need to worry about solubility
in alcohol.
IIRC the actual hydration process chemically consumes about 25% of the cement weight in water, but water is also bound physically in the gel phase.
For concretes a water/cement-ratio of 0.4-0.6 is usually employed, but this is determined by workability / flow properties and not chemical
consumption. But even then the concrete is kept wet after the initial setting to ensure enough water for complete curing. I would try an experiment
with alcohol in excess to determine how much water is consumed under these conditions.
Most cement kilns are indeed used to incinerate wastes and hazardous materials, but the temperatures involved virtually guarantees that no organics
are left in the product. Heavy metals will of course remain, but I doubt there will be more than trace amounts due to stringent environmental and
occupational hazard requirements.
Hexavalent chromium (usually from trace amounts in the ore or metal wear in the mills) can represent a real hazard to cement workers (contact
dermatitis), at least in northern Europe this is reduced by adding ferrous sulfate to the milled cement.
Consumption is of course out of the question. You do not add industrial grade chemicals to anything meant for human consumption.
[Edited on 4-2-16 by Fulmen]
We're not banging rocks together here. We know how to put a man back together.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Fulmen  | | I would try an experiment with alcohol in excess to determine how much water is consumed under these conditions. |
That'll be for someone else to explore.
I just needed to make dry ethanol for the TCA synth.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Fair enough. Nevertheless an interesting approach, and like many good ideas it's pretty obvious once you start to think about it. I know refluxing
with CaO can be used to get to 99.5%, but cement should do much of the same and it's far more available.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
diggafromdover
Hazard to Self
 
Posts: 84
Registered: 24-2-2015
Location: New Hampshire
Member Is Offline
Mood: Inconherent
|
|
Did you evaporate any of the product to determine the weight of dissolved material? If so, how much was there, and can you speculate on it's
composition?
Enjoying second childhood with REAL chemistry set.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
More ways to skin a . . .
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by diggafromdover  | | Did you evaporate any of the product to determine the weight of dissolved material? If so, how much was there, and can you speculate on it's
composition? |
That's quite a good idea, actually. Just evaporating a teaspoon and looking for solid residue could potentially be revealing.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Nice find hissingnoise.
Today the ethanol got dunked in cement again, for 3 hours this time.
Didn't dry it completely, as the test with pot permanganate shows 
Left is the treated ethanol, right the untreated. Barely any percieveable difference.

The still-not-dry ethanol was tested (left to right) with silver nitrate, potassium hexaferrocyanate and barium chloride.

Not sure what the yellow stuff means, yet there seem to be no chloride or sulphate ions present.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
excellent work. I've been roasting small amounts of oyster shell for CaO. Portland MUCH easier.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Oysters are a bit too expensive for my taste - i'm more of a cement-and-chips man 
Edit:
(the CaCO3 => CaO + CO2 thing requires 800+ C)
[Edited on 4-2-2016 by aga]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
I have the ground shell fer my chikins
*edit* Can't stand the thought of EATING an oyster
[Edited on 2-4-2016 by arkoma]
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Not sure what the yellow stuff means, yet there seem to be no chloride or sulphate ions present. |
Almost certainly that the hexaferrocyanate is insoluble in EtOH!
You need longer drying times (but NEVER, EVAH listen to me!  ), as hissing's page
suggests. ), as hissing's page
suggests.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Be the First Billy in the History of Your People to eat one! Lead Them to the Promised Land! Yee-Haw!
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I agree, more time is needed. Vogel's procedure with CaO is 6hours of refluxing and standing over night.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The surprising thing is that nobody has jumped in to say 'I always dry my ethanol like this ...' and detailed their usual process.
It is hard to imagine that i'm the first amateur chemist ever to physically dry ethanol (as distinct from 'knowing what to do')
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by aga  | The surprising thing is that nobody has jumped in to say 'I always dry my ethanol like this ...' and detailed their usual process.
It is hard to imagine that i'm the first amateur chemist ever to physically dry ethanol (as distinct from 'knowing what to do')
|
I use the CaO method in Vogel to get to 99.5%, then 3A mole sieves to get very near 100%.
I make CaO by calcining slaked lime, Ca(OH)2, available dirt cheap by the lb at my local weed & feed store. Bring your own container. This is
also available as pickling lime at my grocery store.
I calcine the Ca(OH)2 in my muffle furnace at ~700°C, IIRC, until constant weight is achieved. Likely 1-2hrs does it.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
| Pages:
1
2 |