esgeroth
Harmless

Posts: 4
Registered: 1-1-2016
Member Is Offline
Mood: No Mood
|
|
Making bismuth crystals, but they won't oxidize.
Anyone here do anything with bismuth? I don't know if this is the best place to get help with this but I sure don't know of any forums dedicated to
bismuth so here it goes.
My son and I have been having lots of fun making crystals from different metal salts and other things. So we wanted to try making some crystals from
bismuth due to its very colorful oxidation layer. I bought a small amount on ebay and we made a couple of small crystals that looked great. I wanted
to make something a little bigger so then I bought 5 pounds of bismuth that was advertised as 99.99% pure.
I melted it down, let it cool for a few minutes, and poured it out. It left some crystals in the container but did not oxidize so it does not have any
color to it at all. Just silver. Also some of the crystals are a bit different looking as well. Some are forming triangles instead of squares and
there aren't as many of the larger cube shapes. I've tried melting it several times with the same results.
From any of the videos I have watched it seems that this is pretty easy to do. Is it likely that my bismuth is contaminated with something else? I got
it from a reputable dealer that specializes in bismuth alloys. But the bismuth I bought should be 99.99% pure. I don't think it would have been
contaminated when I melted it since it went straight from the bag it came in into a ceramic baking dish I had been using to melt it.
So does anyone have any tips on working with this stuff or should it be easier than this?
 
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
I'm not sure if it's this case but I was making bismuth crystals and results were really nice.But after I accidently contamined my bismuth with very
little amount of tin, every attempt ended similarly to yours.
Now I will probably buy new bismuth and try it again.
This is one of my best bismuth crystals (the "price 20€" doesn't belong to crystal it's just coincidence if it interests anyone ). ).

[Edited on 25-2-2016 by crystal grower]
|
|
|
esgeroth
Harmless

Posts: 4
Registered: 1-1-2016
Member Is Offline
Mood: No Mood
|
|
How did you contaminate your bismuth? Was it just from the container it was in? Did you see the small triangular crystals as well? It sure seems like
that's what has happened to mine but it went straight from the bag it came in into the ceramic dish I used to melt it.
Anyway, here's some of what I was able to get before I bought this 5 pound block. I just wish I could replicate it now.
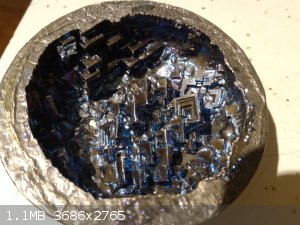 
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
No, when I bought bismuth it was very pure.
It´s long story how i have contamined them so nevermind  . .
at least it was only 250g bismuth that is contamined.
In the first image you can see nice crystals of pure Bi.
the second one is contamined bismuth (and yes, there are triangles as u said).
 
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
hkparker (another SM member) and I talked about this a while back - he had the same problems you are having. He sent his Bi to someone to get it
assayed and it did indeed come back as contaminated with a significant amount of tin. I believe he did some experiments and the amount of tin
necessary to inhibit oxide formation was very low. So your bismuth may be lower purity than advertised! Purity has a huge impact on oxide colors and
crystal formation. My 99.99% Bi forms lovely blues and greens, but my 6N (99.9999%) gets into reds and yellows. Really beautiful, but too precious to
experiment with!
|
|
|
esgeroth
Harmless

Posts: 4
Registered: 1-1-2016
Member Is Offline
Mood: No Mood
|
|
Yeah, that's the same type of crystals as I have been getting from this stuff. It definitely seems like it must be contaminated. I don't see where I
could have introduced anything into it since I melted it in a ceramic container. Plus its probably a lot harder to contaminate 5 pounds of the stuff
rather than 250g. I asked the seller about it and he even sent me the analysis from their distributor certifying it as 99.99% pure.
Is it expensive to get something like this assayed? Where can this be done?
|
|
|