blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Element Ionisation energies: a trend anomaly?
I found this interesting question on a prominent physics forum.
If you look at the 1st Ionisation Energies of s and p-block elements we know that energy goes down with increasing Period number. We all know why too.
But if you look at the trend for several d-block groups it doesn't hold. The following graph illustrates the anomaly:
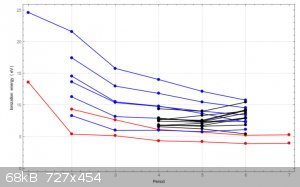
s-block groups (Groups 1 and 2) are in red, p-block (Groups 13 to 18) are in blue and several d-block groups (but not all are shown) are in black.
Anyone willing to shed some light on this conundrum?
[Edited on 29-2-2016 by blogfast25]
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I am going to offer an explanation that is kind of my go-to for all transition metal chemistry and is undoubtedly a gross simplification. But it does
go somewhere to explaining this phenomenon as well as variable oxidation states.
Basically the d block elements have multiple options for stable electron arrangement.
http://www.ptable.com/#Orbital
Clicking along the 3d, 4d and 5d elements shows anomalies in the order that shells are filled (Cr is the first -- it is more stable as 4s1-3d5 rather
than the expected 4s2-3d4.) The number of these anomalies increases as you go down the table. (Check Cr, Cu on the first row. Nb, Mo, Ru, Rh, Pd, Ag
on the next row. Third transition row seems to be pretty well-behaved but that might be a simplification too. One would want to compare the electron
configuration of the ion formed as well.)
It follows that if these elements find a lower energy state than the one expected by following the "normal" pattern, then those electrons will be more
difficult to remove leading to a higher first ionisation energy.
It would be insightful to look at all 10 groups in the d block and see what behaviours emerge. Without checking the ionisation energies, I think it
is probably a transition metal thing rather than a d-block thing.
[edit]
clarification.
[Edited on 29-2-2016 by j_sum1]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
The second and third periods of d block elements have just added a chunk of nuclear charge and a full f shell. f shell electrons are poor at
shielding nuclear charge. Google "Lanthanide contraction".
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@j_sum1 and Marvin:
Thanks, that solves it, I think.
j_sum1: not sure what you mean by:
| Quote: | | I think it is probably a transition metal thing rather than a d-block thing. |
|
|
|
annaandherdad
Hazard to Others
  
Posts: 387
Registered: 17-9-2011
Member Is Offline
Mood: No Mood
|
|
The "Chromium glitch", where an electron is borrowed from the 4s-subshell to the 3d-subshell on going from Vanadium to Chromium, is due to the fact
that you lower energy by having a half-filled subshell (in this case, 4d; Cr's 5 4d electrons make it half full). The reason is that the all the
spins can be aligned, hence you have a spin symmetric state, hence a spatially antisymmetric state, hence keeping the charges away from one another.
This is an issue having to do with the symmetries of the electron wave functions, rather than effectiveness of screening. Cr actually has two
half-filled subshells, both 4s and 3d.
When you add the next electron after the subshell is half filled, it raises the energy because the new electron cannot be spin aligned with the ones
already there, and the spatial part of the wave function is forced to be more symmetric, thereby raising the Coulomb energy of the electrons among
themselves.
Any other SF Bay chemists?
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I very much respect annaandherdad 's posts and that is an excellent answer, but perhaps to a different question. I read the OP twice and the second
time realised this was about trends going down a group, not across a period.
I think the full picture is probably complex but to the best of my education I'm sticking with screening as the major contribution.
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by blogfast25  |
j_sum1: not sure what you mean by: | Quote: | | I think it is probably a transition metal thing rather than a d-block thing. |
|
I meant that the zinc group might revert to the normal pattern since by my reasoning, having full d orbitals, it lacks possibilities for alternative
electron arrangements.
Really, my explanation is little more than a hand-wave and one I use with students to explain variable oxidation states. I need to be able to explain
why Fe forms 2+ ions and 3+ ions and not 8+ ions. At the level that my students need, I simply point out that the transition metals have multiple
options for stable electron configuration.
Thanks annaandherdad for a more complete explanation. Is the "chromium glitch" a technical term? I am going to use it anyway.
Marvin, you may well be closer to the truth with your explanation. I know about the lanthanide contraction. But I did not know about the
diminished electron shielding afforded by f electrons. But, as you say, it is undoubtedly complex. More complex in fact than the data blogfast
posted. I am just assembling some more complete data. I will post soon.
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Here's the data.
It seems that the stronger pattern here is the depression in ionisation energy of the second transition row.
Undoubtedly more than one thing going on here.
Attachment: First IOnisation of D block elements.xlsx (14kB)
This file has been downloaded 295 times
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
From row 4 (numbering so that row 4 starts with K) to row 5, the ionization energy decreases, which is normal, for every element pair except
for vanadium[3d3,4s2]/niobium[4d4,5s1], chromium[3d5,4s1]/molybdenum[4d5,5s1], and nickel[(3d8,4s2 + 3d9,4s1)/√2] /palladium[5d10]. All of these
show the d-block anomaly which I assume is due to electron degeneracy in the d-block. I'm pretty sure the actual physics is similar to (but not the
same as) the energy-level depression in resonant bond systems:
https://en.wikipedia.org/wiki/Resonance_%28chemistry%29#Hist...
| Quote: | | In the classical system, the coupling produces two modes, one of which is lower in frequency than either of the uncoupled vibrations; quantum
mechanically, this lower frequency is interpreted as a lower energy. |
In the same way, some multiple-electron states of the d-block can "resonate" (in a mathematical sense) to allow a lower frequency (energy) eigenstate,
which increases the ionization energy. The trend is also clear when comparing the reactivity of aluminum and gallium, or phosphorus and arsenic.
The increase in ionization energy from row 5 to row 6, on the other hand, can be easily explained by the lanthanide contraction.
[Edited on 1-3-2016 by clearly_not_atara]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks j_sum1.
|
|
|
annaandherdad
Hazard to Others
  
Posts: 387
Registered: 17-9-2011
Member Is Offline
Mood: No Mood
|
|
Marvin, you're right, of course, I didn't answer the question that was asked. I was just commenting on the general situation, and trying to make the
point that the electron symmetry is part of the problem when computing the state of lowest energy (as well as screening).
Ultimately the calculations are heavy numerically, and some of the rules that have been developed (eg Hund's rules) are partly empirical.
Another point that is sometimes misunderstood is that the whole concept of an electron configuration is only an approximation. The true ground state
wave function of the atom is not exactly a product of single particle orbitals (what an electron configuration would imply). Instead, the electron
configuration is the best approximation one can make to the true wavefunction by such a product.
Chromium glitch is my own terminology.
jsum1, very cool interactive periodic table link, thanks.
Any other SF Bay chemists?
|
|
|