| Pages:
1
..
17
18
19 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Particle in a 2D circular box with zero potential energy (Ct'ued)
4. Solving the angular equation:
$$\frac{Y''}{Y}=-m^2$$
$$Y''+m^2 Y=0$$
With two complex roots, +im and –im this has the general solution:
$$Y(\theta)=c_1\cos m\theta + c_2\sin m\theta$$
θ is of course an angle, in radians, and an angle ‘repeats’ itself every 2π. For that reason we can choose either c<sub>1</sub>
or<sub>2</sub> to be zero. We choose the latter:
$$c_2=0 \implies Y=c_1\cos m\theta$$
Again, because θ is an angle the following condition must also be met:
$$Y_m(\theta)=Y_m(\theta+2\pi ) \implies \cos(m\theta)=\cos(m(\theta+2\pi))=\cos(m\theta+2m\pi)$$
$$\implies m=0, \pm 1, \pm 2, \pm 3,...$$
The negative values are trivial because:
$$\cos(|m|\theta)=\cos(-|m|\theta)$$
The function needs to be normalised, with the normalisation requirement:
$$\int_0^{2\pi}Y^*Yd\theta=1$$
Y<sup>*</sup> is the complex conjugate of Y. But Y is a Real function, so:
$$Y^*=Y \implies Y^*Y=Y^2$$
$$\int_0^{2\pi}Y^2d\theta=\int_0^{2\pi}c_1^2\cos^2(m\theta) d\theta=1$$
$$\implies c_1=\frac{1}{\sqrt{\pi}}$$
$$Y_m=\frac{1}{\sqrt{\pi}}\cos m\theta$$
There’s one exception, for m=0:
$$m=0 \implies Y_0=c_1$$
$$\int_0^{2\pi}c_1^2d\theta=1 \implies c_1=\frac{1}{\sqrt{2\pi}}$$
$$Y_0=\frac{1}{\sqrt{2\pi}}$$
So we have a fully defined solution for Y(θ), as well as values for m:
$$m=0,1,2,3,...$$
[Edited on 27-6-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yay ! morganbw's post caused the next page to appear.
it was taking ages for page 18 to render.
Edit:
Hold on hoss.
So we're in Polar co-ordinates now and we got a Derivative, then we got a derivative of a derivative.
In plain english, what's the first derivative mean ?
The rate of change of the angular rotation ?
[Edited on 27-6-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Edit:
Hold on hoss.
So we're in Polar co-ordinates now and we got a Derivative, then we got a derivative of a derivative.
In plain english, what's the first derivative mean ?
The rate of change of the angular rotation ?
|
Sorry, only just saw your edit.
Firstly, both polar coordinates and Cartesian coordinates are completely valid ways to define the position of a point (the position does not change in
function of the coordinate system used).
Derivatives (first, second or whatnot) can be used in both systems, the same rules of derivation apply.
A derivative like:
$$\frac{\partial u(r,\theta)}{\partial r}$$
... is the rate of change of the wave function (in polar coordinates) vis-a-vis the r-component of the position (r,θ).
| Quote: | | In plain english [...] |
That's a perfectly legitimate question but hard to answer. Mathematical statements like the Schrödinger equation are jam packed full of logic. But
that makes 'unpacking' the parcel into intelligible spoken or written language quite hard.
Incidentally, the Schrödinger equation used here is the time-independent one. It is free of time and therefore cannot be used to determine velocities
or angular velocities (both are derivatives to time). For these we need to apply quantum operators on the wave function.
I'll wait for your response before solving the radial part of the SE and then we'll be able to start plotting some functions!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. It was a silly question really now i'm sober enough to think about it.
It's a bit like asking what a 5-dimension co-ordinate set actually means ...
Please, continue.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Particle in a 2D circular box with zero potential energy (Ct'ued)
5. Solving the radial equation:
So far we have:
$$r^2R''+rR'+(k^2 r^2-m^2)R=0$$
With boundary condition:
$$u(R,\theta)=R(R)Y(\theta)=0 \implies R(R)=0$$
We make a simple substitution:
$$\rho=kr$$
Minimal reworking then yields:
$$\frac{d^2R(\rho)}{d\rho^2}+\frac{1}{\rho}\frac{dR(\rho)}{d\rho}+\Big(1-\frac{m^2}{\rho^2}\Big)R(\rho)=0$$
This is a Bessel Differential Equation, a type of DE that also pops up in the solution of the hydrogen SE, the Fourier (heat) equation and other
important PDEs.
It has a solution for every m, called a Bessel function: J<sub>m</sub>:
$$m \to J_m(\rho)=J_m(kr)$$
Where:
$$R(\rho)=J_m(\rho)=\displaystyle \sum_{n=0}^{+\infty}\frac{(-1)^n}{n!\Gamma(n+m+1)}\Big(\frac{\rho}{2}\Big)^{2n+m}$$
The J<sub>m</sub> functions are sophisticated infinite term polynomials and they look like this:
J<sub>0</sub> compared a cosine function, to show the resemblance:
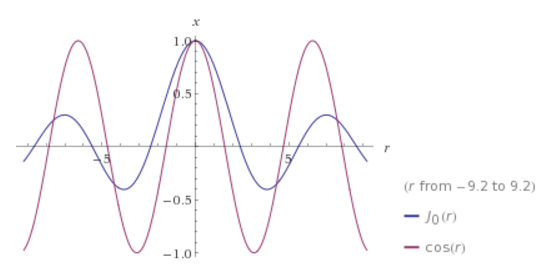
J<sub>3</sub> compared a sine function, to show the resemblance:
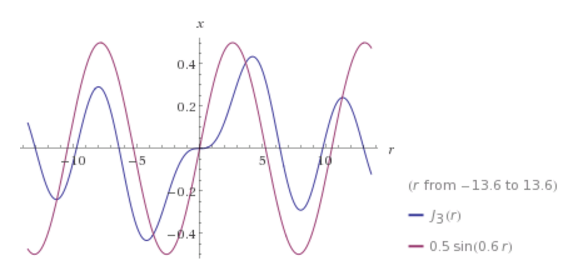
Later we’ll make use of these resemblances to define some quasi-solutions, because the actual Bessel functions really don’t lend themselves easily
to graphical 2D plotting.
Radial wave function quantisation:
The particle in a 2D circular box is a quantum system and its wave functions and energy levels are quantised. Quantisation is derived from the
boundary condition:
$$J_m(kR)=0$$
Basically this means that for the roots (zero-points) z<sub>m,n</sub>, where n designates the n<sup>th</sup> zero of
J<sub>m</sub>:
$$k_{m,n}R=z_{m,n}$$
The z values can be found here:

(Source).
$$k_{m,n}=\frac{z_{m,n}}{R}$$
Much higher up we stated:
$$k^2=\frac{2mE}{\hbar^2}$$
We can now use that expression to derive the energy spectrum of the system:
$$E_{m,n}=\frac{\hbar^2}{2mR^2}z_{m,n}^2$$
And the ground state energy:
$$E_{0,1}=\frac{\hbar^2}{2mR^2}(2.4048)^2$$
<hr>
Next we'll replace the Bessel functions with appropriate sines/cosines and the plotting can begin!
[Edited on 29-6-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Particle in a 2D circular box with zero potential energy (Ct'ued)
6. Quasi-solutions of the radial equation - plots:
Two cases need to be considered:
1. m = 0
$$R(r)=c_1\cos(kr)$$
$$R(R)=c_1\cos(kR)=0 \implies kR=\frac{n\pi}{2}$$
$$n=1,3,5,7,...$$
$$k=\frac{n\pi}{2R}$$
$$R_0(r)=c_1\cos\Big(\frac{n\pi r}{2R}\Big)$$
Normalisation condition to obtain c<sub>1</sub>:
$$\int_0^Rc_1^2\cos^2\Big(\frac{n\pi r}{2R}\Big)rdr \implies c_1=\frac{2}{R}$$
$$R_0(r)=\frac{2}{R}\cos\Big(\frac{n\pi r}{2R}\Big)$$
2. m = 1,2,3,...
$$R(r)=c_2\sin(kr)$$
$$R(R)=c_2\sin(kR)=0 \implies kR=n\pi$$
$$n=1,2,3,...$$
$$k=\frac{n\pi r}{R}$$
$$R_m(r)=c_2\sin\Big(\frac{n\pi r}{R}\Big)$$
Normalisation condition to obtain c<sub>2</sub>:
$$\int_0^Rc_2^2\sin^2\Big(\frac{n\pi r}{R}\Big)rdr \implies c_2=\frac{2}{R}$$
$$R(r)=\frac{2}{R}\sin\Big(\frac{n\pi r}{R}\Big)$$
Putting it all together:
$$u(r,\theta)=R(r)Y(\theta)$$
m = 0:
$$u_0(r,\theta)\approx \frac{2}{R\sqrt{2\pi}}\cos\Big(\frac{n\pi r}{2R}\Big)$$
$$n=1,3,5,...$$
m = 1,2,3,...
$$u_m(r,\theta)\approx \frac{2}{R\sqrt{\pi}}\sin\Big(\frac{n\pi r}{R}\Big)\cos(m\theta)$$
$$n=1,2,3,...$$
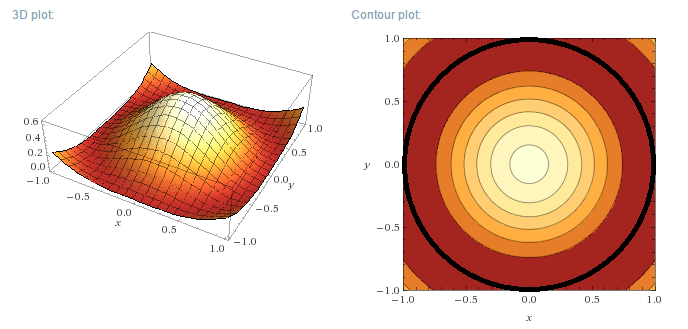
Plotting the functions:
Instead of plotting the values of the wave functions u, the probability density functions will be plotted, for a circular well of R=1 m. As u is a
Real function, the probability density function is simply:
$$\rho=u_m^2(r,\theta)$$
(not to be confused with the previously used rho)
Of course, R=1 m is not a realistic value for a quantum system. For a more realistic size of say 1 nm (1x10<sup>-9</sup> m) the vertical
axis values would have to be multiplied by 1x10<sup>18</sup> m<sup>-2</sup>.
Left (next post) are the 3D plots, right the contour plots, with the actual boundary indicated as a thick black line. In the contour plots, areas of
high probability density are marked as light colours and values close to zero as red.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Particle in a 2D circular box with zero potential energy (Ct'ued)
m=0, n=1
m=0, n=3
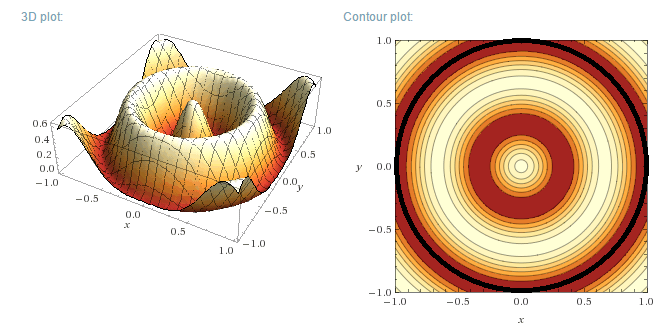
m=0, n=5
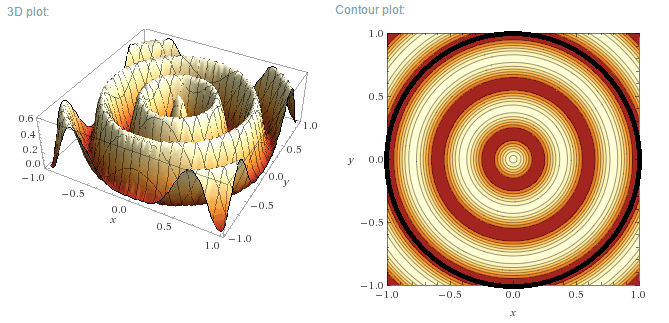
m=1, n=1
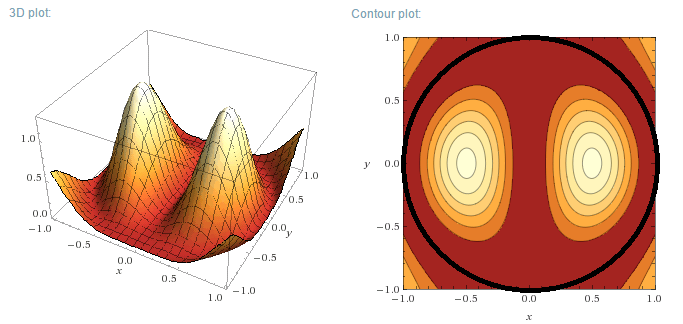
m=1, n=2
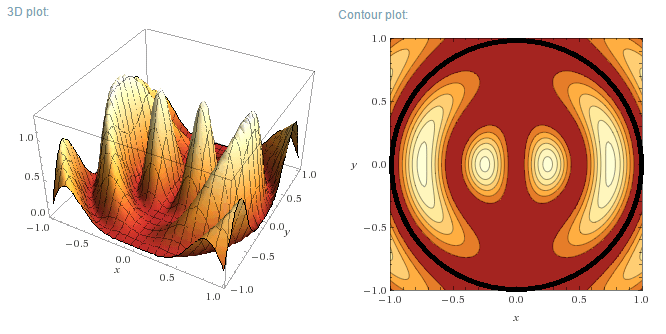
To come: m=2, m=3
[Edited on 30-6-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Particle in a 2D circular box with zero potential energy (Ct'ued)
m=2, n=1
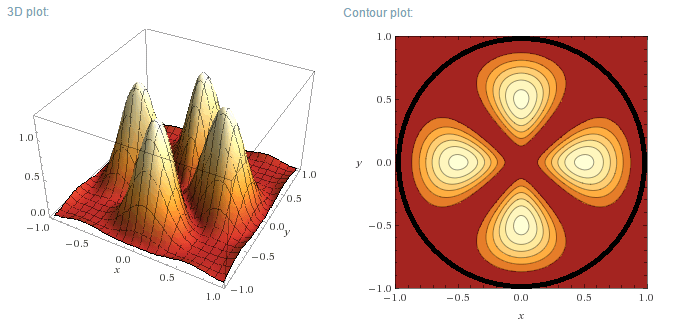
m=2, n=2
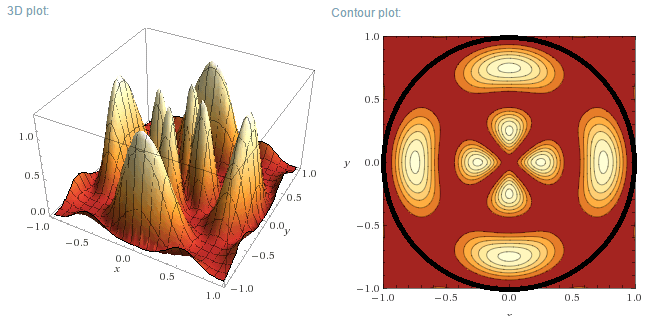
m=3, n=1
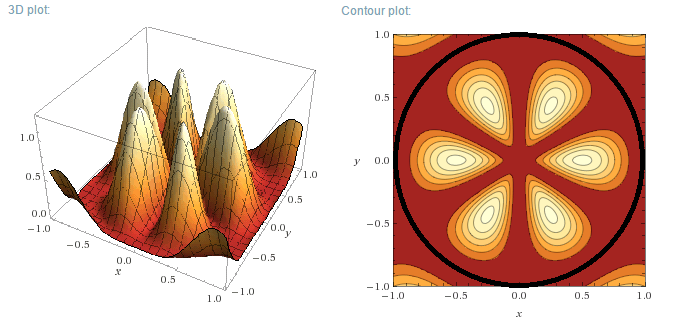
m=3, n=2
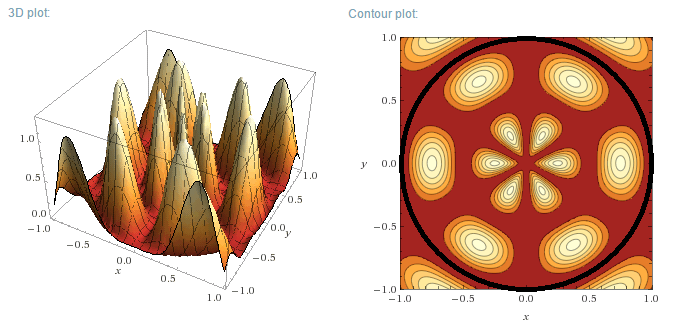
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Blogfast25:
After titillating me with your color rendered 3D graphs, I am waiting for your orbital derivations. I have even fired up a cobbled together xp pc to
run my old copy of mathcad so I might give them a try.
[Edited on 27-7-2016 by wg48]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48  | Blogfast25:
After titillating me with your color rendered 3D graphs, I am waiting for your orbital derivations. I have even fired up a cobbled together xp pc to
run my old copy of mathcad so I might give them a try.
|
What specific orbital derivations are you looking for? I would certainly be interested in assisting with that! 
Recently I tried 3D plotting of the H 2pz orbital but something went wrong. I suspect the error was due to the conversion from polar to
Cartesian coordinates. Unfortunately neither matlab nor Wollram's plotting function allow direct plotting of polar functions in 3D.
For now I leave you with the contour plot of the actual wave function (not squared), which also shows the zero-lines (nodal lines), for the system
above (m=3, n=2). All straight lines and circles are nodes where the wave function changes sign:
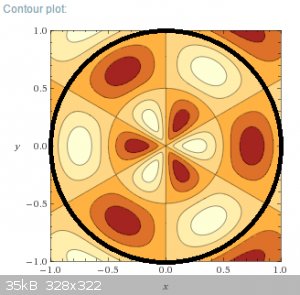
And here's a fascinating tidbit. The energy quantisation of the particle in a circular well with infinite walls is essentially chaotic. And this
explains why a circular flexible membrane, like a drum for musical purposes, has no fundamental tone or well defined harmonics. It's basically noise.
[Edited on 27-7-2016 by blogfast25]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by wg48  | Blogfast25:
After titillating me with your color rendered 3D graphs, I am waiting for your orbital derivations. I have even fired up a cobbled together xp pc to
run my old copy of mathcad so I might give them a try.
|
What specific orbital derivations are you looking for? I would certainly be interested in assisting with that! 
Recently I tried 3D plotting of the H 2pz orbital but something went wrong. I suspect the error was due to the conversion from polar to
Cartesian coordinates. Unfortunately neither matlab nor Wollram's plotting function allow direct plotting of polar functions in 3D.
For now I leave you with the contour plot of the actual wave function (not squared), which also shows the zero-lines (nodal lines), for the system
above (m=3, n=2). All straight lines and circles are nodes where the wave function changes sign:
And here's a fascinating tidbit. The energy quantisation of the particle in a circular well with infinite walls is essentially chaotic. And this
explains why a circular flexible membrane, like a drum for musical purposes, has no fundamental tone or well defined harmonics. It's basically noise.
[Edited on 27-7-2016 by blogfast25] |
I had no particular orbital in mind. My interest was just general curiosity. In part motivated by how an object with some symmetry can have modes
with a different symmetry. I have now sorted that out now.
I thought drums do have a distinct modes but I suspect you mean something different than that. I will reread your particle in box to see if I can
understand what you mean or even better play with it in mathcad.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48  |
I thought drums do have a distinct modes but I suspect you mean something different than that. I will reread your particle in box to see if I can
understand what you mean or even better play with it in mathcad.
|
Look here:
http://www.sciencemadness.org/talk/viewthread.php?tid=65532&...
$$k_{m,n}R=z_{m,n}$$
The roots of the Bessel function (zm,n) are the quantum numbers of the system.
The PDE for a circular, elastic membrane is, bar the coefficients, the SAME as for the particle in a circular box. In that derivation the
zm,n are basically the frequencies of oscillation: the spectrum is inharmonic. Drum beats are noise.
Now look at the vibrating modes of the membrane:
https://en.wikipedia.org/wiki/Vibrations_of_a_circular_membr...
Ring a bell? 
Let me know what I can do to help with orbital plotting: it would be a first here at SM! 
<hr>
Some excellent sources of orbital representations:
http://www.st-andrews.ac.uk/physics/quvis/simulations_chem/c...
And:
http://winter.group.shef.ac.uk/orbitron/
[Edited on 27-7-2016 by blogfast25]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Ok I think I understand what you mean now.
Personally I would not use the term noise ( or chaotic) to describe the spectrum of a drum in this technical context.
In practice the modes of a drum are excited preferentially by where you hit the drum and by the resilience of the drum stick.
As for ringing a bell: the whealing sounds of bells is caused by the anharmonic relationship between its fundamental and overtones. The fundamental
decays faster than some overtones producing a sound that chances frequency, the walling. LOL
Thanks for the offer of help. I may get round to it some time. I am presently improving my attenuation graph and trying to work out how I can get
mathcad to calculate masses from a chemical equation. I am stuck at inputting the atomic weights and abbreviations at the moment.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48  | Ok I think I understand what you mean now.
Personally I would not use the term noise ( or chaotic) to describe the spectrum of a drum in this technical context.
In practice the modes of a drum are excited preferentially by where you hit the drum and by the resilience of the drum stick.
|
Chaotic and noise in the sense of inharmonic.
The amplitude spectrum (relative weight of the various frequencies) is indeed determined by the initial condition:
$$u(r,\theta,0)=f(x,y)$$
|
|
|
| Pages:
1
..
17
18
19 |