SmellNoEvil
Harmless

Posts: 15
Registered: 23-2-2016
Member Is Offline
Mood: No Mood
|
|
Pimelic Acid Synthesis?
I would like a -Preferably not too difficult- method of synthesizing pimelic acid for making cyclohexanone.
I have looked through the patents cited on the Wikipedia page, and all of them either call for using an autoclave or aluminum isopropoxide, which I
don't have.
If there is a simpler method of preparing pimelic acid or cyclohexanone, I would like to know what it is.
Thanks!
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
PVC pipe cement tends to contain a fair bit of cyclohexanone (10-30%) and it isn't too expensive. Workup isn't what I'd call fun, but it's doable.
Here is a sample SDS: http://www.oatey.com/msds/sds-us--oatey-pvc-heavy-duty-clear...
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
pimelic acid can be made from salicylic acid according to wiki
http://orgsyn.org/demo.aspx?prep=CV2P0531 ,see "(B) from salicylic acid"
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 126
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
I afraid pimelic acid preparation more difficult and expensiv than make cyclohexanone from phenol.
|
|
|
SmellNoEvil
Harmless

Posts: 15
Registered: 23-2-2016
Member Is Offline
Mood: No Mood
|
|
Doesn't making cyclohexanone from phenol require hydrogenation and a suitable catalyst such as palladium,while making pimelic acid doesn't?
[Edited on 22-3-2016 by SmellNoEvil]
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 126
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
No, you can prepare cyclohexanol in 2 step. 1 step : phenol hydrogenisation with cheap Ni-catalysator to cyclohexanol, (170-180 C) and then this
oxidize by bleach to cyclohexanon. Very easy.
Julius B. Cohen: Practical Organic Chemistry 1908 (181. page)
L. Gattermann: Laboratory Methods of Organic Chemistry Completely Revised By Heinrich Wieland Transslated from Twenty-Fourth German Edition New York
The Maximillian Company 1937 (379. p.)
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
If you're up to it, cyclohexanone can also be prepared from phenol in a single step with excellent yields via a Benkeser reduction. As long as the
hydrolysis step at the end is done correctly, yields should be around 90-95%. A Benkeser reduction is basically just a more powerful version of a
Birch reduction, and is usually done at slightly higher temperatures. You'll need lithium metal and a solvent like methylamine, ethylamine or
ethylenediamine, as lithium in regular ammonia is not going to be strong enough to reduce the phenoxide ring. Lithium is readily obtained from lithium
batteries, and methylamine is easily synthesized from hexamine, which can be bought as fuel tablets at just about any camping/outdoor store.
Anyway, it's just another route to consider. The only real challenge is drying the freebase methylamine gas and condensing it into a liquid. But other
than that, it's pretty simple and straightforward. If the meth cooks can do these kinds of reductions, I'm sure you can. If nothing else, it will make
for an interesting write-up. I don't think anyone else here has actually attempted to make cyclohexanone this way. The only reason I even know about
this route is because I randomly stumbled across it while reading some literature on Birch reductions a while back.
If you're interested, here's how the reduction seems to work:
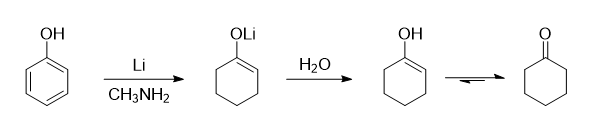
The phenol is initially reduced by lithium all the way to the enolate shown above. Water is then added to quench the reaction and protonate the
enolate, giving a vinyl alcohol. The enol form then quickly tautomerizes to the more stable ketone form to give cyclohexanone.
Reference: A Comparison of Methods Using Lithium/Amine and Birch Reduction Systems
Page 16 of the .pdf, bottom paragraph, left side:
"Furthermore, phenol and β-phenylethyl alcohol have been reduced under Benkeser conditions. Thus, phenol is converted to cyclohexanone in 96% yield
by lithium in methyl- or ethylamine provided the hydrolysis of the reaction mixture is carried out rapidly with little lithium remaining. The
cyclohexanone presumably arises from tautomerization of vinyl alcohol 81."
|
|
|
skip
Hazard to Self
 
Posts: 54
Registered: 16-5-2015
Member Is Offline
Mood: No Mood
|
|
PVC purple primer has the ketone you want, and indeed work-up is a bitch. distill off the lower boiling compounds then add water and azeotrope out the
ketone. Form the bisulfite compound to clean it up. Basify to seperate the ketone oil and redistill. If you distill with out the water there is some
kind of azeotrope forming and it won't distill as clean. You can buy cyclohexanone by the gallon off eBay, or by the qt. Its easy to get.
|
|
|