Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
benzidine
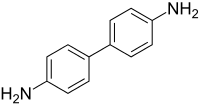
Today I synthesized benzidine HCl using a 2X scale version of the procedure in Brewster (forum library). This synthesis uses nitrobenzene as
precursor. The reductant is hydrogen generated via Mg turnings and methanol. Although the nitrobenzene charge was only 5.2 ml the sequential
hydrogenations (-->azobenzene -->hydrazobenzene) generated a lot of heat. Lastly, benzidine is formed as a rearrangement of hydrazobenzene. As
advised I used a tub of cold water for cooling when it looked like my reflux condenser was being overwhelmed. By restraining the 500ml RBF/reflux
condenser at just one point it was easy to keep the reactants mixed by swirling the whole system.
Tomorrow I will freebase (I guess that's the term) my product to benzidine using 5% NaOH, crystalize, filter, dry, weigh, then calculate a yield.
Below are some pictures I took along the way:
Attachment: php6umGgL (90kB)
This file has been downloaded 1109 times
hydrazobenzene heated to 75-85°C

acidified hydrazobenzene

aqueous benzidine HCl
[Edited on 25-3-2016 by Magpie]
[Edited on 25-3-2016 by Magpie]
[Edited on 25-3-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Very nice. I've wanted to make a small amount of benzidine for some time (given the uniqueness of the rearrangement reaction and for production of
congo red).
If this goes to prepub, I think a significant disclaimer about the product's carcinogenicity, proper handling, and waste management is in order.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by UC235  | Very nice. I've wanted to make a small amount of benzidine for some time (given the uniqueness of the rearrangement reaction and for production of
congo red).
If this goes to prepub, I think a significant disclaimer about the product's carcinogenicity, proper handling, and waste management is in order.
|
Yes, that's why I'm making it: as a precursor for Congo Red
The carcinogenicity issue kept me from making this for some time. It's everywhere with the Congo Red precursors. I feel I have good control of my
lab hygiene so decided to go for it. After all, benzene is carcinogenic. I don't know how you can do much organic chemistry without it, IMO.
But if I decide to place this in prepub I would add the warnings.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
It's because all the old Orgsyn preps use it as an indicator and you can't buy it easily, isn't it. Because that's why I want to make it and it seems
like we have the same list of plans usually.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
yes - that's it.  Plus it looks like a neat synthesis. Plus it looks like a neat synthesis.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
I guess a lot of us are working on the same synthesis. I currently have some recrystallized 1-nitronaphthalene from last weekend's work sitting on my
lab bench. I was planning to convert it to naphthionic acid this weekend if I have the time, though I haven't prepared the requisite benzidine yet.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Not to mention, that benzidine is insanely overpriced as a chemical. At least I found 2-3x prices on a mass to mass basis compared to other
"similarly not too complicated" compounds. (What can be considered similar is a personal taste though.) :-)
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
So, rather than use a different indicator like bromophenol blue or methyl orange, you decided to make one of just a handful of chemicals that have
been banned from (most) commercial manufacture.
Well- it's not the decision I'd have made...
Did it occur to you that there was a reason the price was so high?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
As unionised has mentioned, there are alternatives to congo red as an indicator with very similar range and transition pH. I'm not sure how bad congo
red itself is, but benzidine (and its derivatives) is/are one of the COSHH banned substances, at least here in the UK.
Perhaps the warnings are overexaggerated as in the case with benzene (the IARC monographs deal with exposure in an industrial setting), but its still
worthwhile avoiding where safer alternatives exist, and taking due care if you decide to persist.
It is however an interesting synthesis once used as an undergraduate practical, and the product is indeed used alot as an indicator in OrgSyn preps.
For those interested I have attached an old J. Chem. Ed. paper detailing the preparation
Attachment: Preparation of congo red.pdf (2.3MB)
This file has been downloaded 743 times
[Edited on 26-3-2016 by DJF90]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This whole issue of carcinogeneity and what chemicals we should or should not be using needs some vetting, IMO. I hope we can do this on this board.
FYI it is interesting to look at the International Agency for Research on Cancer (IARC) list 1 carcinogens. Note that ethanol and UV radiation (which
I assume includes the UV of sunlight) are on the list, along with benzidine: IARC 1
[Edited on 26-3-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
"IARC MONOGRAPHS VOLUME 99" is on the internet and it mentions all the benzidines research. It shows a link to bladder cancer. Similar perhaps to
popular boogeyman hexavalent chromium, in the effect of dust on unprotected workers shoveling in the packaging department for 10 years being
considered as dangerous by the regulation/litigation culture as looking at or even thinking of a 0.1% solution.
BTW treatment of the dye with dithionite regenerates benzidine; this would be a convenient synthesis if the dye was as available as it was.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The aqueous benzidine HCl described above in the OP was freebased to benzidine. This required taking the slurry to a litmus paper blue endpoint using
210 ml of 5% aqueous NaOH.

aqueous benzidine slurry
The somewhat gummy product was then caught on a 7cm Buchner funnel and dried. It was drying very slowly at room temp so I placed it in a drying oven
at 80°C for a couple hours.
This solid product was then dissolved in a little (10-15ml) 95% ethanol heated to boiling. The flask was then cooled over ice to recrystallize. The
dried benzidine product is shown below:

benzidine
Results
The yield was 1.9g for a %yield of 41.2%. mp was 121.5°C. The Wiki mp range is 122-125°C.
Discussion
I suspected that the yield would not be too good based on some reading. That is why I doubled the scale of the Brewster procedure. I wanted to get
at least 1g.
As mentioned above benzidine is an IARC list 1 carcinogen. Therefore this procedure should not be performed without adequate safety precautions and
PPE.
The mp was hard to determine because the solid is a dark gray. It may well have melted over the Wiki range indicated. The 121.5°C is just the first
indication of darkening and slumping that I noted. At any rate I am confident it is pure enough for my purposes.
Questions, comments, and suggestions are welcomed.
[Edited on 27-3-2016 by Magpie]
[Edited on 27-3-2016 by Magpie]
[Edited on 27-3-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Very nice write-up, as usual Magpie.
I'm quite intrigued by:
Quote: Originally posted by Magpie  |
This synthesis uses nitrobenzene as precursor. The reductant is hydrogen generated via Mg turnings and methanol. |
Mg turnings and methanol generate H2 at significant rates? Or did I miss something...
Also, there seems to be a C-C coupling reaction going on. Any reaction mechanism known?
[Edited on 27-3-2016 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for your interest, blogfast.
Mg and methanol produce H2 like gangbusters. Another advantage of Mg (over Fe or Zn) is that my turnings were very thin and light so almost floated.
But the azobenzene-hydrazobenzene and methoxide products become very viscous during the reaction. I kept them fairly well mixed by vigorous manual
swirling. At the same time I was keeping the slurry at 75-85°C, so heavy gloves are required.
I really don't know what was generating all the heat. It's hard to believe it was the reaction of the 5.2 ml of nitrobenzene to azobenzene then to
hydrazobenzene. Could it have been the heat of formation of methoxide and H2?
I haven't seen the mechanisms for the first two products. The mechanism for the rearrangement to benzidine (a classic organic puzzle) is shown in the
Wiki for benzidine: rearrange
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  |
Mg turnings and methanol generate H2 at significant rates? Or did I miss something...
Also, there seems to be a C-C coupling reaction going on. Any reaction mechanism known? |
Reaction of Mg with alcohol can indeed be aggressive, but may need initiation. See Klute's prep of salicylaldehyde for a photo (http://www.sciencemadness.org/talk/viewthread.php?tid=10124)
That being said, I think it is extraordinarily unlikely that the hydrogen itself is the reducing agent but rather that the magnesium reduces
nitrobenzene through a single electron transfer mechanism and the coupling to azobenzene or azobenzene-N-oxide occurs between (radical?) intermediates
which are further reduced.
The strong-acid catalyzed rearrangement of hydrazobenzene to benzidine (Benzidine Rearrangment) is a classic mechanistic mystery, thought likely to be
a concerted [5,5] sigmatropic reaction.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks, Magpie.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Very interesting thread, the chemistry is interesting, the mechanism is, and the cancer risk discussion are all topics I am interested in.
As for the cancer risk, I have always thought that many risks were overrated, but still am careful with some compounds due to common sense. Max
Gergal made at least 8 of the 10 top known carginogens in bulk and he is now well over 90. Many scientists I know used benzene, chloroform, and
others for years and are fine, but that is not a scientific fact or a real study. But should anyone be interested, I have many IARCpublications, as
part of the books that I recently got. So if anyone wants to study cancer risks, I can help you find the data, although some may be online now, it
appears.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by UC235  | | That being said, I think it is extraordinarily unlikely that the hydrogen itself is the reducing agent but rather that the magnesium reduces
nitrobenzene through a single electron transfer mechanism and the coupling to azobenzene or azobenzene-N-oxide occurs between (radical?) intermediates
which are further reduced. |
You're right about the reduction proceeding through an SET mechanism; however, the coupling to azobenzene most likely occurs through nitroso and
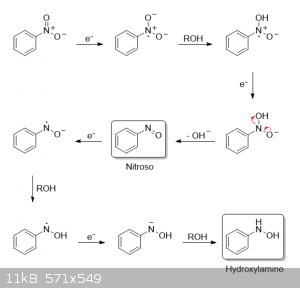
hydroxylamine intermediates, not collisions between nitrogen radicals. I've always found these dissolving-metal reductions rather interesting
mechanistically. And since I had some time to kill, I drew a possible mechanism for the reduction of nitrobenzene to nitroso and hydroxylamine
intermediates, along with their coupling to azobenzene and subsequent reduction to hydrazobenzene. The mechanism for the final rearrangement of
hydrazobenzene to benzidine can be found on Wikipedia, as already mentioned.
Since the reduction is done in absolute methanol, I assume that the methanol is functioning as the proton source. I will say that this reduction was a
bit trickier to figure out than those done in acidic media. Anyway, if anyone's interested, here's what I came up with:
Reduction of nitrobenzene to nitroso and hydroxylamine intermediates:
Coupling to azobenzene and reduction to hydrazobenzene:
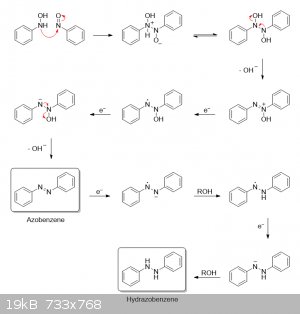
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
@Darkstar: Very nice. Thanks for this.
Are the e- running loose in the solution?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
It's certainly possible. Unfortunately, there haven't been a great deal of studies done on the actual electron-transfer mechanism of these
magnesium-alcohol reduction systems, so it's difficult to really say one way or another at the moment. With that said, it was recently discovered that
at least sodium in ethanol does in fact generate solvated electrons, and polar solvents are known to readily trap free electrons. Solvated electrons
have also been found to exist in water as well, sometimes referred to as "hydrated electrons."
[Edited on 4-14-2016 by Darkstar]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I understand that Na dissolved in liquid ammonia produces solvated electrons that cause the solution to take on a blue tint.
I was wondering if one could expect this same color to result from the reaction of Mg with methanol. The solutions in my benzidine synthesis are so
colored that any faint blue would be masked, I assume.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I would imagine that the methanol is too reactive for the concentration of solvated electrons to ever really get high enough to give the
characteristic blue color. Ammonia works much better as a solvent because it's fairly resistant to reduction by the electrons, allowing for a much
more stable and concentrated solution.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Online
Mood: Big
|
|
^^^ It seems like the azoxy compound should be an intermediate:
http://orgsyn.org/demo.aspx?prep=CV2P0057
In particular when you consider that hydroxylamines are the reduction product using acids (Zn/NH4Cl or borane) and azo coupling products are the
products in strongly basic conditions, one possible explanation is that the radical anion:
Ph-N*-O(-) <<--->> Ph-N(-)-O* <<--->> Ph*=N-O-
is very resistant to reduction when deprotonated, but is reduced to the hydroxylamine when protonated. The anion can dimerize to azoxybenzene by
eliminating the equivalent of O2-, possibly involving a H+ abstraction from the solvent.
Alternatively, if k1*[PhN*O-][PhN*OH] > k2*[e-][PhN*OH], you get azo coupling, otherwise the reverse, for some rate constants k1, k2.
[Edited on 15-4-2016 by clearly_not_atara]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I agree. I was even going to show an azoxy intermediate in my condensation mechanism as well. In the end, though, I chose to omit it to save steps.
But in reality, I imagine the intermediate shown in the fourth step of my second mechanism would immediately get deprotonated to an azoxy given the
strongly basic conditions. The excess magnesium would then transfer an electron to the azoxy to give a negative charge on the left nitrogen and a
radical on the right nitrogen, with the radical center being stabilized by the two adjacent negative charges and the aromatic ring (in addition to
resonance):
The left nitrogen then abstracts a proton from methanol to give a radical nitroxide, followed by a second electron transfer to the radical nitrogen to
give an anion. The oxygen finally grabs a proton and gets eliminated as hydroxide, along with the extra proton on nitrogen to give azobenzene:

| Quote: | In particular when you consider that hydroxylamines are the reduction product using acids (Zn/NH4Cl or borane) and azo coupling products are the
products in strongly basic conditions, one possible explanation is that the radical anion:
Ph-N*-O(-) <<--->> Ph-N(-)-O* <<--->> Ph*=N-O-
is very resistant to reduction when deprotonated, but is reduced to the hydroxylamine when protonated. The anion can dimerize to azoxybenzene by
eliminating the equivalent of O2-, possibly involving a H+ abstraction from the solvent. |
While I don't deny the possibility of dimerization, another explanation for the lack of azo coupling under acidic conditions is the protonation of the
hydroxylamine's nitrogen. This would prevent the hydroxylamine intermediates from being able to attack the nitroso intermediates, effectively
inhibiting their condensation to azo products. Thus strongly basic conditions would promote condensation to an azoxy, while strongly acidic conditions
would hinder it. Also, acidic reductions only tend to stop at the hydroxylamine stage when weak acids are used. When strong acids are used, the
reductions will usually go all the way to an amine. (that, or an oxime that gets hydrolyzed to a ketone, depending on the substituents on the nitro
group carbon)
In the case of phenylhydroxylamine, the nitrogen is much more basic than the oxygen. The reason nitrobenzene reductions using zinc in aqueous
NH4Cl give phenylhydroxylamine and not aniline is likely because the conditions simply aren't acidic enough to protonate the oxygen over
the nitrogen, especially if the nitrogen is already protonated. Reduction of hydroxylamines to amines under acidic conditions is thought to involve an
initial protonation of the hydroxyl group, which then leaves as water, and a subsequent reduction of the resulting nitrenium ion.
But as I said, I don't discount the possibility of dimerization through radical coupling, either as a competing mechanism alongside condensation, or
as the primary pathway to azoxybenzene itself.
[Edited on 4-16-2016 by Darkstar]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I posted here an article about the study of rearrangements of N,N'-diarylhydrazines. It is mostly about the rearrangement of these into semidines, but
might be interesting nevertheless.
|
|
|