Romix
Hazard to Others (Literally)
  
Posts: 427
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Bases as solvents, metals
I understand what happens when acid reacts with metal.
Halogen gains electrons, releasing hydrogen.
But what happens when base acts as a solvent.
For example Al + NaOH. Hydrogen released here too.
What about ammonia solutions with copper? No hydrogen in this reaction, but it dissolving. Same for nickel but much much slower.
[Edited on 2-4-2016 by Romix]
[Edited on 2-4-2016 by Romix]
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Only metals that are amphoteric in their lowest stable state of oxidation react with alkalis. Hydroxide ion gains electrons, metal gives them
resulting in the metal oxide and hydrogen, oxide then reacts with another hydroxide ion giving a metalate (or hydroxometalate) ion.
Smells like ammonia....
|
|
|
brubei
Hazard to Others
  
Posts: 187
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
Pourbaix diagramm gives the state of a metal in different conditions
for exemple: aluminium is corroded in aqueous solution at pH < 4 and pH > 9 while H2O gives H2 or O2
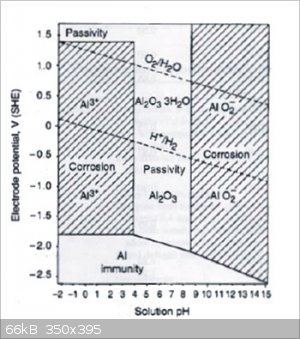
In non acidic condition, corrosion is given by the couple
O2(g) + 2 H2O + 4 e- ⇄ 4 OH-(aq) E = +0,40V
As you can see there is no gaz generated.
Then copper is oxidized to oxide or hydroxide (see area pH > 7 Eh/V > 0.4, corresponding to aqueous ammonia)

Then ammonia give complexe with non-valent electron and produce soluble compounds
[Edited on 2-4-2016 by brubei]
By the same way nickel is oxidized in basic aqueous condition, but it tend to produce NiO a solid acting as a protection against oxydation (
Ni(OH)3 is out of the domain of H2O stability)
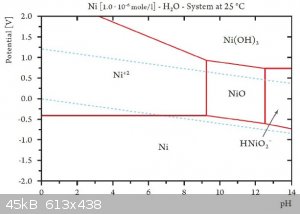
[Edited on 2-4-2016 by brubei]
[Edited on 2-4-2016 by brubei]
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 427
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by brubei  | Pourbaix diagramm gives the state of a metal in different conditions
for exemple: aluminium is corroded in aqueous solution at pH < 4 and pH > 9 while H2O gives H2 or O2
In non acidic condition, corrosion is given by the couple
O2(g) + 2 H2O + 4 e- ⇄ 4 OH-(aq) E = +0,40V
As you can see there is no gaz generated.
Then copper is oxidized to oxide or hydroxide (see area pH > 7 Eh/V > 0.4, corresponding to aqueous ammonia)

Then ammonia give complexe with non-valent electron and produce soluble compounds
[Edited on 2-4-2016 by brubei] |
Copper corrodes, and ammonia dissolves its oxide?
Maybe.
[Edited on 2-4-2016 by Romix]
[Edited on 2-4-2016 by Romix]
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 427
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by brubei  |
By the same way nickel is oxidized in basic aqueous condition, but it tend to produce NiO a solid acting as a protection against oxydation (
Ni(OH)3 is out of the domain of H2O stability)
|
Yes, right. And it's black.
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 427
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Oxygen needed in both reactions.
|
|
|