lamura
Harmless

Posts: 4
Registered: 7-9-2006
Member Is Offline
Mood: No Mood
|
|
CaCl2: please help me.
is there someone that could give me a reference on the decomposition temperature of CaCl2?
the melting temp. is 772 C then I expect something like 850-900 C...is it possible?????
please, help me!!!!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
If it melts, I doubt it decomposes (I may be mistaken). It could certainly boil though.
Tim
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Excerp........But calcium chloride is very stable, and its decomposition in this way is incomplete, and requires enormous expenditure
of heat, besides that used in evaporating the solution of calcium chloride to dryness.
.........................source,
http://www.lenntech.com/Chemistry/chlorine-industry.htm
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
tumadre
Hazard to Others
  
Posts: 171
Registered: 10-5-2005
Member Is Offline
Mood: No Mood
|
|
plasma temperatures required to decompose it, I boiled it with a carbon arc, didn't smell any CL2
|
|
|
lamura
Harmless

Posts: 4
Registered: 7-9-2006
Member Is Offline
Mood: No Mood
|
|
CaCl2
Hi to all,
thank you for your replies, but it is clear that after melting the next important temperature is the boiling one.
For my pourposes it's important to know just the decomposition temperature, because I have to force the Ca to react with Carbonium (decomposed from a
methane flux) without forming CaC2 end then it is important that the decomposition temperature of CaCl2 could be less then the temperature at which
CaC2 is favourized.
In alternative, do you know another salt that releases Ca when it dicomposes???? The decomposition temperature must be less then 800 C.
This is the problem.
thanks to all
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Calcium oxalate or azide(might be too rapid) will decompose to calcium under quite low heat. Much less than 800.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
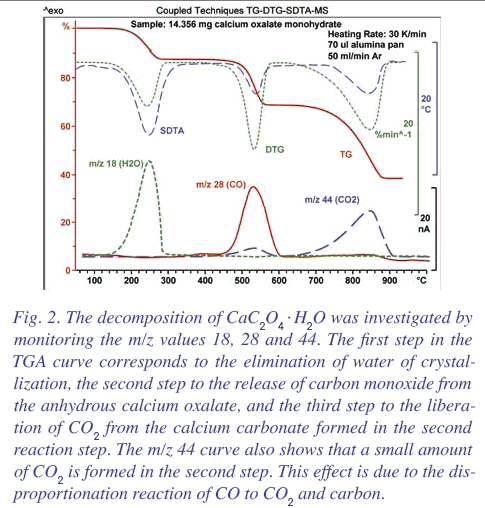
Calcium oxalate surely can't decompose to calcium. I thought it was generally known that it decomposes to CaCO3 and CO. Then at higher temperature,
the CaCO3 decomposes to CaO and CO2.
DTG diagram with the decomposition product analysis:
According to this paper Ca(N3)2 and other metal azides do decompose to the metal and N2, but explosively so. 
CaH2 does not explode when decomposed to Ca and H2, but mixtures of H2 with air or other oxidants can explode. 
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Ca and air or other oxidants can explode, too, what's your point? 
Tim
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Thanks Nicodem, I just figured it would decomose the same as several heavy metal oxalates, guess not.
Calcium azide just decomposes without explosion(deflagration?) above 110C and lower than 160C, greater than 160C it explodes.
EDIT: Does not CaH2 explode in contact with water? Much more violently then an alkali metal. Source: an old professor of mine.
[Edited on 9-9-2006 by rogue chemist]
|
|
|
lamura
Harmless

Posts: 4
Registered: 7-9-2006
Member Is Offline
Mood: No Mood
|
|
o my god!....and there is no way to evoid explosion???
the procedure should be the following (one time that the good Ca-salt has been find for this pourpose)
Silicium substrate (with a thin oxide layer : Si/SiO2) and the salt should dissolved in some solution (ethanol, methanol, ...) to drop it on the
substrate. After the solvent is evaporated, the substrate will be put into a quartz furnace.
In a continuous flux of argon (to purge the system) and after methane the heater can be turned on.
The goal is that heating decompose decompose the salt and the methane in order to let the Ca atomes to play the role of catalyst toward Carbon atoms
(without forming CaC2!!!).
thank you
gian
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
So you want to....catalyze SiO2 + 2C = 2CO + Si or Si + C = SiC, with calcium? Is calcium a dopant?
Does methane dissociate appreciably at the temperature you desire? I get the feeling it doesn't. Don't you need a plasma or something to get active
methyl bits?
And as I mentioned elsewhere ...why not vapor deposit Ca in a vacuum chamber, if available?
Tim
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 202
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
C'mon guys, he's trying to grow large crystals of sparkly moissanite or semiconductor wafers 
[Edited on 10-9-2006 by FrankRizzo]
|
|
|
goob
Harmless

Posts: 1
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by rogue chemist
Calcium azide just decomposes without explosion(deflagration?) above 110C and lower than 160C, greater than 160C it explodes.
[Edited on 9-9-2006 by rogue chemist] |
what is calcium azide exactly? CaN3? the azide ion is confusing..
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by goob
what is calcium azide exactly? CaN3? the azide ion is confusing.. |
Read the rest of the thread. The rational formula was mentioned and a paper discussing the salt was linked.
|
|
|