Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Oxidation of aromatic side-chains at the benzylic carbon?
Is there a regioselective method to halogenate or hydroxylate the side-chain of an unsubstituted aromatic at the benzylic position? For instance, how
would you prepare 1-phenylethanol or 1-bromo-1-phenylethane from ethylbenzene? What if the substrate were propylbenzene?
I believe that ethylbenzene would be simple and would involve a low temperature 1,2-addition from an acid-catalyzed hydration of styrene, but what
about when the side-chain is extended? What generalization could be used for both to still target the benzylic carbon. I know the benzylic carbon is
vulnerable to oxidation, so I know that would have something to do with it and involve further oxidation at that position.
Am I incorrectly assuming that bromination of ethylbenzene would occur at the least substituted carbon, which would be the terminal end? If
this is the case, then a radical bromination with Br2/UV would give one of the desired product.
I know the product is less expensive than the starting material and that acetophenone is a starting material for 1-phenylethanol as all that is
needed is to reduce the double bond. I also know that the alcohol can be made from the halogenated compound. It's the mechanisms that I am interested
in discovering. I am working through my books from college, and this is the result of that.
EDIT: Changed 1-bromo-phenylethane to 1-bromo-1-phenylethane to add missing position.
[Edited on 12-4-2016 by Loptr]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I am seeing search results on Google talking about hydrogen peroxide, dichromate, etc. as an oxidizer for the preparation of acetophenone from
ethylbenzene.
Would these sorts of approaches work for alkylbenzenes with longer side-chains without resulting in cleavage?
It seems I need to refresh on the reactivity of alkyl carbons in general. The rate at which the halogenation of the various carbons is listed below,
and it turns out the benzylic carbon would be the first to become substituted.
Carbons with one or more aryl substituents (benzylic positions) react faster than:
Carbons with three alkyl substituents (tertiary positions), which react faster than:
Carbons with two alkyl substituents (secondary positions), which react faster than:
Carbons with one or zero substituents (primary positions)
[Edited on 12-4-2016 by Loptr]
|
|
|
MeshPL
Hazard to Others
  
Posts: 329
Registered: 20-4-2015
Location: Universe
Member Is Offline
Mood: No Mood
|
|
Generally speaking, chlorination and especially bromination quite selectively prefer benzylic carbons. Especially ones using NBS, but I think you can
brominate ethylbenzene at alpha position by radical mechanism as well with good results.
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
What is funny is that I actually found this same problem in an OC book I picked up for $3 entitled "Organic Chemistry" by Hepworth, Waring, and
Waring. I don't know how good the book is, but I found this example in it, so it was worth something.
[Edited on 13-4-2016 by Loptr]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Free-radical halogenations (especially brominations) are selective towards the benzylic position because the intermediate benzylic radical is
stabilized through resonance by the adjacent aromatic ring. Since radical carbons at the benzylic position only have partial radical character they
are less reactive and thus easier to form.
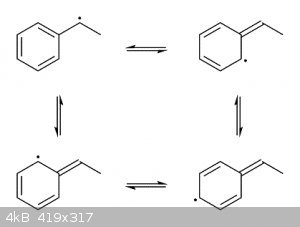
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Yes, you are right.
There are two positions where a hydrogen atom can be abstracted by a halogen radical, and forming two possible radicals. The first being the benzylic
radical, which as you mentioned mention is favored due to the stability of resonance interactions with the benzene ring, which isn't possible with the
other potential radical product. Therefore, alpha-substitution is favored and predominates.
You would just need to make sure you used a low concentration of halogen at any given time to ensure the second halogenation didn't occur to product
the 1,2-dihalo compound.
Thank you! This was very helpful. It's nice to finally have some time to actually sit down and go back through my books.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
However, I can't see how this would prevent a bromination to occur onto the ring, at least as a secondary product. There is no steric hindrance and it
seems equally plausible.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Eddygp  | | However, I can't see how this would prevent a bromination to occur onto the ring, at least as a secondary product. There is no steric hindrance and it
seems equally plausible. |
Could you explain?
I know that benzylic halogenation is a radical mechanism, while halogenation of the ring is an electrophilic mechanism, so under these conditions the
ring shouldn't be touched.
It was my understanding the ring halogenation needed a catalyst, such as iron, to produce FeX3 (X=Cl,Br) in-situ or that it required reflux with UV
light present, in which case you would end up with halogenation at every carbon on the ring, instead of mono-bromination as can be seen with the
catalyst. That last statement applies to benzene. I am not sure of the effect the alkyl group has on ring bromination, other than it being a
directing group.
[Edited on 13-4-2016 by Loptr]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Quote: Originally posted by Loptr  | Quote: Originally posted by Eddygp  | | However, I can't see how this would prevent a bromination to occur onto the ring, at least as a secondary product. There is no steric hindrance and it
seems equally plausible. |
Could you explain?
I know that benzylic halogenation is a radical mechanism, while halogenation of the ring is an electrophilic mechanism, so under these conditions the
ring shouldn't be touched.
It was my understanding the ring halogenation needed a catalyst, such as iron, to produce FeX3 (X=Cl,Br) in-situ or that it required reflux with UV
light present, in which case you would end up with halogenation at every carbon on the ring, instead of mono-bromination as can be seen with the
catalyst. That last statement applies to benzene. I am not sure of the effect the alkyl group has on ring bromination, other than it being a
directing group.
[Edited on 13-4-2016 by Loptr] |
Hmmm, true, my bad. However, how are you intending to create the radicals in this case?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Step 1. Initiation
Heat or uv light cause the weak halogen bond to undergo homolytic cleavage to generate two bromine radicals and starting the chain process.
Step 2. Propagation
(a) A bromine radical abstracts a hydrogen to form HBr and a benzyl radical, then
(b) The benzyl radical abstracts a bromine atom from another molecule of Br2 to form the brominated product and another bromine radical, which can
then itself undergo reaction creating a cycle that can repeat.
Step 3. Termination
Various reactions between the possible pairs of radicals allow for the formation of Br2 or the brominated product. These reactions remove radicals and
do not perpetuate the cycle.
[Edited on 13-4-2016 by Loptr]
[Edited on 13-4-2016 by Loptr]
[Edited on 13-4-2016 by Loptr]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Eddygp  | | However, I can't see how this would prevent a bromination to occur onto the ring, at least as a secondary product. There is no steric hindrance and it
seems equally plausible. |
Bromination on the ring would break aromaticity, making it far less favorable than bromination at the benzylic position.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Darkstar  | Quote: Originally posted by Eddygp  | | However, I can't see how this would prevent a bromination to occur onto the ring, at least as a secondary product. There is no steric hindrance and it
seems equally plausible. |
Bromination on the ring would break aromaticity, making it far less favorable than bromination at the benzylic position. |
Bromination as in a substitution reaction, not an addition.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Sorry, I thought he was asking why the ring carbons in the intermediate shown above don't get brominated, as they also have partial radical character
as well.
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
No need to be sorry as it wasn't specified, and bromination, as far as I know, can refer to either substitution or addition, especially if the
substrate has double bonds. In fact, the reaction I mentioned above where refluxing benzene during halogenation would result in all of the carbons
being halogenated, is an addition reaction with benzene that does break aromaticity. Ring delocalization is broken and 1,2,3,4,5,6-hexahalocyclohexane
is produced as the result of all of those carbons becoming halogenated.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Loptr  | | In fact, the reaction I mentioned above where refluxing benzene during halogenation would result in all of the carbons being halogenated, is an
addition reaction with benzene that does break aromaticity. Ring delocalization is broken and 1,2,3,4,5,6-hexahalocyclohexane is produced as the
result of all of those carbons becoming halogenated. |
Do you have a cite for that?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I have seen it referenced in a paper that I came across where it cited an original paper on the work, but all I can find now is its use as an example
of an addition reaction with no reference to the original work.
It's all over the place. One thing I did mistate was that the reaction took place under reflux, well that's not exactly true, it is heat and pressure,
so no where near what is encountered during a normal reflux.
http://chemwiki.ucdavis.edu/Core/Organic_Chemistry/Hydrocarb...
http://crab.rutgers.edu/~alroche/Ch17.pdf
http://www.chemguide.co.uk/organicprops/arenes/halogenation....
I will stop at these three, as there are countless other references to this one reaction. I will see if I can find a reference to the original work to
find the reaction parameters.
[Edited on 14-4-2016 by Loptr]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Actually, I am seeing those sites refer to different parameters. The one from ucdavis just says bubble chlorine though hot (how hot??
too hot for atmospheric pressure; a bomb, maybe? that's misleading) benzene while exposed to UV light.
[Edited on 14-4-2016 by Loptr]
[Edited on 14-4-2016 by Loptr]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Loptr  |
It's all over the place. One thing I did mistate was that the reaction took place under reflux, well that's not exactly true, it is heat and pressure,
so no where near what is encountered during a normal reflux.
|
1,2,3,4,5,6 hexahydrocyclohexane,a.k.a lindane is prepared by commerical chlorination of benzene not reflux.
loptr,please make up your mind on what you want
if you want 1-phenylethanol then - http://pubs.rsc.org/en/content/articlelanding/2001/p1/b00884...
and if you want 1-bromoethylbenzene then -http://www.sciencedirect.com/science/article/pii/S0040403904...
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I want neither, CuReUS. 
I hate being spoonfed, so I typically will ask questions according to my understanding, and not jump straight to what I am actually trying to figure
out. I was working through the process to figure out how a carbonyl group could be attached to the alpha carbon of any alkylbenzene. The problem I was
facing was an incorrect understanding in the reactivities of the carbons, which I incorrectly believed the reactivity would result in a terminal end
halogenation. I was looking for a potential method to either hydroxylate or halogenate, as both could lead to insertion of the carbonyl. Forgive me,
as I am only having just started to try and regain this knowledge after not being used for 10+ years ago. I am finding there is a lot more to it than
I remember. 
I also love how authors will mix aboratory and commercial procedures together without making the distinction for the end-reader. The original
reference I found to this a while back would have had more information on the conditions, but what I read of it in the paper referring to the original
made it sound like a simple reflux led to an addition and breaking of the aromaticity. The aromaticity was broken, but only using an industrial scale
process, which is easier to swallow now that I see it wasn't a mere reflux.
[Edited on 14-4-2016 by Loptr]
[Edited on 14-4-2016 by Loptr]
|
|
|