Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
Passing chlorine gas
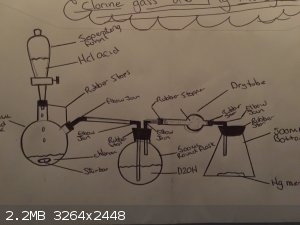
So I've been trying to pass chlorine gas over element Mercury with liquid chlorine and 36% acid and am have no luck what so ever my setup is a 1:1
ratio chlorine drops into acid while been stird with magnetic bar then to a nother flask with water to wash the gas then out into a dry tube followed
into a flask with element murcury problem is I don't see or get any gas just bubbles off chlorine and acid mixing even tried a 2:1 ratio that should
give about 15-20 minutes off green gas I know ther is other ways off doing this but this way seems the safest one for me as its only slightly sol in
water unlike hgCI2 hope someone can help heads farting
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
What do you want? You add acid to chlorine, drive off the chlorine, remove the acid from the chlorine with the water and then remove water to make the
chlorine dry and then pass it over Hg? Why not pass it over Hg directly without all the complicated stuff and the acid?
And you use liquid chlorine for this?
Finally, there is no gas outlet in the final stage. Your entire setup will pressurize strongly and stoppers will pop out of their sockets all over the
setup 
Please tell us what you want. It seems that you want to make HgCl2 from Hg and Cl2, but it is not 100% clear to me.
|
|
|
woelen
|
Thread Moved
19-4-2016 at 01:56 |
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
Hello Woelen am after Hg2CI2 I oready have element murcury about 2kg off the stuff it's for a Al/Hg amalgam I would prefair to clean the gas as a
safety measure yes liquid chlorine 14% Clorex bleach was a option aswell but liquid chlorine is stronger dam I missed the gas outlet out explains why
then  
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I was going to suggest moving the second RBF to the Outlet of the conical flask to neutralise the chlorine ...
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | | I was going to suggest moving the second RBF to the Outlet of the conical flask to neutralise the chlorine ... |
Think that would be a good idea or I could just put a outlet on the end flash cheers mate
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Forgot to say that i'd fill the scrubber pot with NaOH solution.
Just better to neutralise chlorine rather than vent it, especially if you have neighbours.
I did have some success with a 'balloon' when chlorinating sulphur, so the chlorine did not need so much scrubbing or venting (or as much produced).
http://www.sciencemadness.org/talk/viewthread.php?tid=62254
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
I don't have neighbours but I do have a squirty bottle at hand to spray around the room miner leaks sometimes Huds a bit old
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
sigh.
A lack of respect for deadly chemicals tends to work out badly for the over-confident.
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
I wear a respirator mate and a chemical suit
|
|
|
Sulaiman
International Hazard
    
Posts: 3559
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Every time that I see the title for this thread I can't help thinking
https://drawception.com/pub/panels/2014/3-30/4Pt2qnbD2O-6.pn...
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
Well all have to start somewhere I guess lol
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
What is "liquid chlorine"? I know you don't mean liquefied chlorine gas. Pool chemicals vary greatly by brand and type, so whatever you're using might
have stabilizers or simply won't react the way you think it will.
|
|
|
Texium
Administrator
       
Posts: 4515
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Every. Single. Time.
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | | What is "liquid chlorine"? I know you don't mean liquefied chlorine gas. Pool chemicals vary greatly by brand and type, so whatever you're using might
have stabilizers or simply won't react the way you think it will. |
sodium hypochlorite sol'n (Clorox bleach)
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Ah... no I understand your setup. Liquid chlorine is something VERY different from bleach! Bleach is not liquid chlorine, it is a solution of sodium
hypochlorite in water.
Liquid chlorine is a yellow liquid, boiling at -35 C or so. It can be purchased in small lecture bottles, but for private persons it will not be easy
to purchase. If you have access to dry ice, then you can make liquid chlorine yourself, but that is not something for the beginner.
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
I have been following this process by atomicdog from Mercurous Chloride is a simple mercury salt with the form of Hg2Cl2. The reaction described herin
was thought to produce Mercuric Chloride (HgCl2) instead, which is used for making amalgams. But it turns out that Mercurous chloride does a perfectly
fine job for amalgams and is thus a good substitute. Hg2Cl2 is insoluble in alcohol and only slightly soluble in water but it still manages to coat
aluminum in a sol'n of either solvent. It is suggested that Hg2Cl2 amalgams be made in mildly acidic water (pH 5-6) because it is at least somewhat
soluble in water and acidity increases that solubility. Apparently even plain Hg in acidic water can work but Atomic feels that this is very difficult
to measure out plain mercury and thus get the same results. This reaction is super easy and takes only a few hours at most.
On the plus side, Mercurous Chloride isn't quite as dangerous as HgCl2. That insolubility makes it difficult for your body to absorb, the Merck even
says that if ingested, a saline laxative should be administered to prevent mercury poisoning, try that with HgCl2! (joking, don't try that). Hg2Cl2 is
a white, odorless, tasteless powder. Protect it from sunlight or it will slowly degrade into HgCl2 and Hg. Hg2Cl2 sublimes at 400-500C without
melting. Please note that any solution of mercury should be considered EXTREMELY dangerous and poisonous and be handled using vynal gloves with
extreme care.
We'll be making Hg2Cl2 today by passing chlorine gas over elemental mercury at room temperature and pressure. You'll need elemental mercury from an
old thermometer or thermostat, and muriatic acid (31.5% HCl), and sodium hypochlorite sol'n (Clorox bleach). Atomic has found that a 2:1 ratio of
bleach to acid is about right or you could use liquid chlorine (stronger stuff) from the same pool store you got the acid at go 1:1. Chlorine gas is a
nasty green gas that will drive you out of the lab in very small amounts and is so corrosive that in time it will turn steel into dust. You don't want
any leaks in your setup for gas to escape! 200ml of acid and 400ml of bleach will give you 15-20 min of gas and that should do it for you. In an ideal
setup, your Sodium Hypochorite sol'n drips from an addition funnel into a flask filled with your acid and is stirring away. If you don't have mag
stirring then you'll have to swirl the flask occasionally to get everything to react. A tube leads the gas to another where it bubbles through water
to clean the gas and then another filled with CaCl2 to dry it, and then to another where the mercury waits. Atomics experience is that the water and
drying aren't really necessary because not much else will react with the Hg and since Hg2Cl2 isn't really H2O soluble or nightmarishly dangerous that
drying the gas is no big either. But be warned! The best way to go is clean and dry. If you use water to clean the gas be aware that for a few minutes
it will absorb your gas until it becomes saturated and lets it pass through, be patient. When the green gas does hit the mercury you'll se it right
away: Your fast moving, T2 looking blob will turn a dull dark gray instantly and slow down or freeze up entirely. This is a hard layer of Hg2Cl2
forming on the outside of your mercury, protecting it from the gas all around it. You have too pick up the flask and shake that baby to break out the
Hg inside and spread it all around the flask so it can react, expect to be doing a lot of this.
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
So here it is looks bit dirty and not 100% reacted goin to evaporate see what am left with
 
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
Just a update after evaporating still looks gray think it's needs redoing until it Gos White
 
|
|
|
Albert101
Harmless

Posts: 12
Registered: 19-4-2016
Member Is Offline
Mood: No Mood
|
|
Someone anyone lol
|
|
|