kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Guanazoguanazole derivatives
I've tried the synthesis of guanazoguanazole from 2-cyanoguanidine and hydrazine sulphate just mixing 0.1 mol 2-cyanoguanidine and 0.05 mol hydrazine
sulphate (molar ratio 2:1) in water. Cyanoguanidine isn't very soluble in water, but at 50ºC it dissolves completly. I kept the solution stirring at
80ºC for 5 hours.
Once it cools down, a white powder precipitates, which was filtered. The remaining solution should contain guanazole, but ATM I'm more interested in
guanazoguanazole.
350 mg of it was dissolved in 5 ml 20% sulfuric acid. Unlike 2-cyanoguanidine, it dissolves instantly in dilute acid. Sodium nitrite solution was
added dropwise while the solution is in a ice water bath. At the first drop the solution turned orange and a orange powders forms quickly (see
attached image). Gas is evolved (N2 probably), no sign of NO2. I'll add more sodium nitrite until no more gas is evolved, then let it stand at room
temperature and filtrate.

I'll post results, of course.
The resulting orange compound what could be? A nitrosamine? I've seen with guanazole that in some reactions the same nitrite can oxidize the
nitrosamine to a nitramine group, in other papers copper nitrate is used as catalist.
[Edited on 26-4-2016 by kratomiter]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Guanazole was reported by Pellizzari to form a yellow nitroso compound with potassium nitrite, so maybe guanazoguanazole does too. Given the
structure of guanazole (3,5-diamino-1,2,4-triazole) the yellow compound is most likely a nitrosamine but I am not sure whether the nitroso group is on
the azole ring or a one of the amine groups in its tautomeric imino form. What is "guanazoguanazole" though? Without this inofrmation its hard to
suggest anything, are you going to enlighten us?
"I've seen with guanazole that in some reactions the same nitrite can oxidize the nitrosamine to a nitramine group, in other papers copper nitrate is
used as catalist." you say, then give us the references so we can see what the hell you are talking about.
For those who didn't know what guanazoguanazole was check out Roscoe's post about three-quarters of the way down this thread: http://www.sciencemadness.org/talk/viewthread.php?tid=10969#... and download the paper on the chemistry of dicyandiamide V. Interesting read. 
[Edited on 27-4-2016 by Boffis]
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
@Boffis This is the structure of guanazoguanazole (3,5,7-triamino-1,2,4-triazolo [4,3-A]-1,3,5-triazine), posted by @Ritter long time ago:
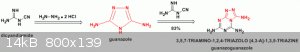
I'm interested in guanazoguanazole because there's very little info about it. It can be a good source of interesting energetic derivatives. I don't
know what reaction happens under diazotation, maybe some cyclation like in aminoguanidine diazotation?
I was wrong about nitrosamine oxidation, I mean diazo intermediate conversion to nitro group, like happens in the synthesis of dinitrotriazole (http://www.prepchem.com/synthesis-of-3-5-dinitro-1-2-4-triaz...).
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The diazotation will likely form on the 3,5 diamino forming a third cyclus (a triazole)
This is seen with 1,2 diamino-benzene (forming a triazopentaring) but also with 1,8-diamino-naphtalene (forming a triazohexaring)...
H2N-C=C-NH2 --HO-N=O--> O=N-NH-C=C-NH2 <==> HO-N=N-C=C-NH2 ==> cyclo (*-N=N-C=C-NH-*) + H2O
The aromatic ring has been left aside for convenience.
H2N-C=C-C(-NH2)=C --HO-N=O--> O=N-NH-C=C-C(-NH2)=C <==> HO-N=N-C=C-C(-NH2)=C ==> cyclo (*-N=N-C=C-C(-NH-*)=C) + H2O
The naphtalenic ring has been left aside for convenience.
The 7 amino may react normally forming a diazonium salt afterwards.
[Edited on 27-4-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Diazonium compounds are not generally strongly coloured, the deep yellow precipitate you illustrated above is more consistent with a nitrosamine or
nitrosimine compound.
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Thank you for both answers. I don't know what compound it is, maybe a nitrosamine, or maybe the cyclation reaction described by PHILOU results in a
conjugated system, which can be a chromophore. I'm going to do some more test, but this product doesn't seem to be an energetic material (at least not
sensitive to shock or flame).
On interesting not is that sulfuric acid is required to obtain it. Performing the diazotation with hydrochloric acid results in the solution changing
to yellow, blue, light green and finally transparent again, evolving lots of colorless gas and some NO2.
Both guanazole and guanazoguanazole seem to form complexes with lead nitrate, resulting in a fine white precipitate. Sadly, with no energetic
properties. BTW, synthesis of DAT from guanazole seems easy but requires lots of solvent to extract it. If DAT forms a complex with lead or other
metal salts, it could be an easy way to isolate it...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Many diazo compounds are colourizers...assuming there is some coupling happening between a free -NH2 and -N=N-OH one may also get a non cyclic
triazole...a triazene (discrete stable form of diazonium)
Ar-N=N-OH + H2N-Ar --> Ar-N=N-NH-Ar + H2O
The typical example 1,3-diphenyl-triazene (diazoaminobenzene) is yellow (see organic synthesis pdf).
So here comes an idea of the final molecule 1,3-bis-guanazoguanazotriazole-triazene
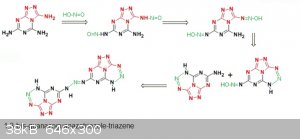
Such large extented planar polyazaaromatic molecules tends to be quite unsoluble... Of course here you have 3 -NH- that induces some acidity, maybe
helping a little solubilisation in basic media.
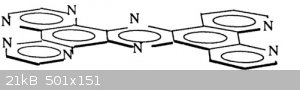
I have worked with PHEHAT (= 1,10-phenanthrolino[5,6-b]1,4,5,8,9,12-hexaazatriphenylene) a fully planar extended polycyclic aromatic molecule and
taking HNRM spectra or making spectrophotometry of it was hard...we had to make use of mixes of acetonitrile and trifluoroacetic acid (deuteriated for
NMR).
The funny part was the synthesis of hexa-amino-benzene (C6(-NH2)6) from trinitrotrichlorobenzene, then trinitrotriaminobenzene and finally a reduction
of all nitros with metallic Na in liquid NH3.
Then condensation-cyclisation-aromatisation of HAB with glyoxal (1/2 equivalents) to get diamino-tetraazaphenanthrene (diamino-TAP).
And finally condensation-cyclisation-aromatisation of diamino-TAP with phenanthroline-dione to PHEHAT...
[Edited on 28-4-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Great job PHILOU!
I've tried the diazotation with of the guanazole solution with the same conditions (sulfuric acid, sodium nitrite and 10º C) and again a orange
precipitate is obtained, it should be the corresponding triazene.
This is also described in this paper in the preparation of 3,5-dinitro-1H-1,2,4-triazole, although they use higher temperatures for this synthesis. They describe lots if
impurities (but not triazenes), specially if DAT is prepared from cyanoguanidine and hydrazine, as I did. One thing that caught my atention is the
presence of azidotriazoles, which makes the HDNT obtained this way more sensitive to shock.
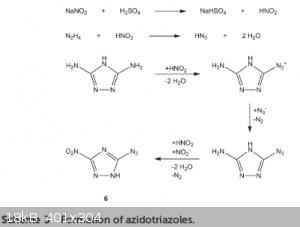
Anyway, isolation of HDNT seem too difficult to me, so I'm not going to try it. In fact, I have no plans for the obtained precipitate.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by kratomiter  | Great job PHILOU!
I've tried the diazotation with of the guanazole solution with the same conditions (sulfuric acid, sodium nitrite and 10º C) and again a orange
precipitate is obtained, it should be the corresponding triazene.
This is also described in this paper in the preparation of 3,5-dinitro-1H-1,2,4-triazole, although they use higher temperatures for this synthesis. They describe lots if
impurities (but not triazenes), specially if DAT is prepared from cyanoguanidine and hydrazine, as I did. One thing that caught my atention is the
presence of azidotriazoles, which makes the HDNT obtained this way more sensitive to shock.
Anyway, isolation of HDNT seem too difficult to me, so I'm not going to try it. In fact, I have no plans for the obtained precipitate.
|
Do you have that interesting paper under hand for me?
The formation of azido compounds is only possible if hydrazine is stil present...a diazido compound might also be considered...usually if wished one
must set the diazonium salt in contact with NaN3...then N2 is set free and replaced by -N3
Ar-N=N-Cl + NaN3 --> Ar-N3 + NaCl + N2(g)
I suspect they used Cu powder (or Cu(NO2)2) for the conversion of the NH2 into NO2 with diazotation and nitrite anion.
[Edited on 29-4-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Of course, I'll send the paper to your mail.
I guess the presence of the azidotriazoles will be minimal because almost all the hydrazine is consumed in the guanazole synthesis. Although I have
azide available, I don't wanna mess with anything so toxic. Also my knowledge in high N compounds chemistry is almost null, I don't understand how
HDNT is formed without Cu (in this paper, in others Cu(NO2)2 is present in the mix).
I'm gonna filter the orange precipitate and do some test just for fun, but I don't believe I'll isolate HDNT or any of its salts.
EDIT: Good news! This time the orange filtrate DETONATED and made a hole on a aluminium foil, it was a tiny amount. 
[Edited on 29-4-2016 by kratomiter]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I requested the dinitro triazole paper, I could see it quickly so I have reposted it in the "recent journal articles of interest" thread.
http://www.sciencemadness.org/talk/viewthread.php?tid=10981&...
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
I did more test. I guess the yellow precipitated is HDNT with some impurities that give it its colour and probably some azidotriazole that make it
more sensitive.
Once dried, the powder keeps its bright yellow colour and can be exposed a few seconds to a flame without any reaction. However, when heating it melts
and a few seconds later detonates. It seems capable to detonated even in the sub-milligram scale, which surprised me. So even without any further
purification it can be an useful explosive, but I need to test shock and friction sensitive.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by kratomiter  | Of course, I'll send the paper to your mail.
I guess the presence of the azidotriazoles will be minimal because almost all the hydrazine is consumed in the guanazole synthesis. Although I have
azide available, I don't wanna mess with anything so toxic. Also my knowledge in high N compounds chemistry is almost null, I don't understand how
HDNT is formed without Cu (in this paper, in others Cu(NO2)2 is present in the mix).
I'm gonna filter the orange precipitate and do some test just for fun, but I don't believe I'll isolate HDNT or any of its salts.
EDIT: Good news! This time the orange filtrate DETONATED and made a hole on a aluminium foil, it was a tiny amount. 
[Edited on 29-4-2016 by kratomiter] |
Thank you for the document in my e-mail.
What is stange is that in the document they convert H2N- into -NO2 efficiently without any Cu (copper)...in this case it seems that upon heating the
diazonium is replaced by a nitrite that reconnect spontaneously to a nitro compound (rare and strange).
Ar-N=N-OH + HO-N=O --> Ar-N=N-O-N=O + H2O
Ar-N=N-O-N=O -heat-> N2(g) + Ar-NO2
Owing to the parenthood of DAT, one may want to investigate melamine, and see if some of the NH2 could be converted by the same process to nitro with
as ultime goal the yet unmade to date trinitro-sym-triazine (nitro-cyanide trimer)
-->nitro-diamino-triazine (and triazene)
-->dinitro-amino-triazine (and triazene)
-->trinitrotriazine
What is also strange is that they mention a red precipitate and NxOy fumes during the initial stage of diazotation of DAT (diamino-triazole), while
you got a yellow-orange one; afterwards their compound seems to be soluble in the media...
Their dinitrocompound is also mentionned as hygroscopic and deliquescent...and apparently water soluble since they extracted the filtrate (liquid
after filtration) with ethylacetate...
So I doubt you got HDNT (acidic dinitrotriazole)...but this might give side reactions to the putative 1,3-bis-guanazoguanazotriazole-triazene pathway
--> some or all -NH2 turned into -NO2.
On its own 1,3-bis-guanazoguanazotriazole-triazene (and maybe some of its Na salts) should be energetic owing to the big N content
--> H3C8N19
--> NaH2C8N19
--> Na2HC8N19
--> Na3C8N19
Many such compounds are endothermic from their elements and display high combustion energy (flame temperature) --> superfuels.
See N#C-C#N and N#C-C#C-C#N; 4525°C and 4990°C oxygen burning flame T° respectively.
One may consider the OB balanced admixture of the Na salt of this with a powerfull oxydiser (Na, K or Li chlorates/Na, K, Li, NH4, N2H5, HONH3
perchlorates/Na, K, Li, NH4, N2H5, HONH3 nitrates)
[Edited on 1-5-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
I think I found the key: the sulfuric acid. I use drain cleaner which have lots of impurities. It doesn't matter in nitrating mixes, but I think is
the reason of the orange precipitate. Performing the diazotation with 20%HCl doesn't produce any precipitate.
The unwashed precipitate detonates when heated (as described previously, I can upload a video if requested) but once washed with water just burns
quickly leaving lots of solid residues, so it has lots of inert impurities. I tried to denate it with some p-DDNP, with no luck obviously.
When I get some diethyl ether or ethylacetate I'm gonna perform the reaction again and the diazotation of both guanazole and guanazoguanazole this
time with HCl and no drain cleaner. I don't understand the mechanism of the reaction, but will be interesting to know if 3,5,7-trinitro-1,2,4-triazolo
[4,3-A]-1,3,5-triazine can be obtained this way from guanazoguanazole.
I also don't undestand how HDNT is hydrosoluble in its protonate form and with 2 nitro groups, which usually add hydrophobicity... I hope engine start
spray will do the job.
Experimenting with melamine sounds exciting, if I find a cheap source I'll try it.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
High acidity makes the hydrosolubility high...because protonation of water occurs...
A-H + H2O <--==> A(-) + H3O(+)
See dinitrophenol, trinitrophenol, trinitroaniline (acidic amine!), nitroform, nitrotetrazole
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Synthesis of (crude) HDNT succesful
Hi!
I've tried again this synthesis, this times following some steps given in Haiges et al (2015) paper. Again, 0.1 mol 2-cyanoguanidine and 0.05
mol hydrazine sulphate (molar ratio 2:1) were dissolved in 40 ml of water at 70 ºC for 7 hours. This time the yield of guanazoguanazole was greater:
the solution seemed supersaturated, so I had to filtrate it twice.
Once filtered, I proceeded to the diazotation of the guanazole solution (I kept guanazoguanazole for later) with an excess sodium nitrite in a ice
water bath. The solution turned instantly orange, it was exothermic and foamed a lot. NOx was evolved.
After stirring 2 hours at 50 ºC, 30 ml of sulfuric acid was added. After standing one hour, the orange compound precipited, so it was filtered and
the filtrated extracted with diethyl ether (3x50 ml).
Once evaporated, the crude product is a yellow oil that deflagates if heated.
As pure HDNT requires vacuum, I'm gonna make the potassium salt.
After all the yield seems low, maybe lots of reactants are wasted forming the orange dye, which doesn't have any energetic property (I think).
I'll post more results once I make the same process with guanazoguanazole.
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
The synthesis of KDNT was a disaster... After dissolving the yellow oil in acetone, 1.5 g of potassium carbonate (I've got no more) were added while
stirring for 1 hour. After filtration and evaporation, the yellow crystals were wet due to the presence of water and sulfuric acid. I know there was
sulfuric acid in there because the crystals (low amount because the yield sucks) deflagated after heating at low temperature and the room was flooded
with SO2 gas 
KDNT appears to be quite sensitive to heat, I could be a good primary if yield was better.
I'm repeating the same process with guanazoguanazole, using ethyl acetate instead of ether and avoiding sulfuric acid (only HCl). I'll post results
shortly.
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
bummer... looking forward to new results!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Aceton is not the best thing to add to make the salt!
1°) Aceton (propanone) is sensitive to HNO3 and HNO2 (from NxOy and H2O) ... as such it will probably destroy any NO providing molecule ... what
H-DNT (dinitrotriazole) is obviously as it is very hydrolysable (*).
During the reaction aceton is nitrosated into CH3-CO-CH2-N=O in equilibrium with CH3-CO-CH=N-OH both very oxydizable.
More likely the -NO2 from H-DNT suffers from nitro-nitrite rearrangement (very frequent when next to electrowithdrawing groups or atoms (see
unstability of hexanitrobenzene, dinitramines, TINGU and trinitro-sym-triazine).
O2N-T-NO2 <----> O=N-O-T-O-N=O (T = triazole)
O=N-O-T-O-N=O + 2 CH3-CO-CH3 ==> HO-T-OH (acid related to cyanuric acid) + 2 CH3-CO-CH=N-OH
2°) Aceton is sensitive to strong acidic media (what H-DNT is) it may:
-form adducts (like (CH3)2C(-DNT)-OH then dehydrate to (CH3-)(DNT-)C=CH2 + H2O and polymerise)
-selfcondense or polycondense into viscous tars.
It is always wiser to ask here before acting and loosing valuable stuff  ... even
if the stuff comes from a mishaps ... even
if the stuff comes from a mishaps
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
I used acetone as described in the paper, maybe the neutralization could be carried in plain ethanol or isopropanol?
The bigger problem for me is the low yield in the synthesis of Guanazole. According to some patens, it should be done with hydrazine dihydrocloride. I
guess the main difference between the sulfate or the dihydrocloride is just the pH, so could the yield be better if the reaction is perfomed in acidic
media?
If the yield is bigger and I get rid of the acid, at least I can obtain a decent amount of KDNT.
BTW, I've got nothing from guanazoguanazole.... At least I want some KDNT to play with!
|
|
|