| Pages:
1
2
3 |
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
Well I'm sure it's some sort of organic varmint. I don't have good photo of it, but I'm still adding a link. https://ctrlv.cz/xQBo
|
|
|
Cucurbit
Harmless

Posts: 17
Registered: 19-3-2016
Member Is Offline
Mood: No Mood
|
|
It looks like little people are swimming around. Just filter them 
[Edited on 7-6-2016 by Cucurbit]
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Cloudy possible organics in Alum crystal growth
Greets,
I have also run into this floating material, filtering helps but it comes back.
I would appreciate info as to whether H2SO4 helped or not, although It does not seem to have affected crystal growth but hard to tell with the
imperfections in the crystal.
Cheers,
Dwarvensilver
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
Well I did not probably add enough to stop that varmint. I'll add some more then post results again.
Btw: I attach few photos of mine chrom alum crystal.
  
|
|
|
Cucurbit
Harmless

Posts: 17
Registered: 19-3-2016
Member Is Offline
Mood: No Mood
|
|
Wow!! Those crystals are magnificent!
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Awesome crystal.
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
Thank you, mine best so far.
Age: 3 months and one week.
|
|
|
Cucurbit
Harmless

Posts: 17
Registered: 19-3-2016
Member Is Offline
Mood: No Mood
|
|
Heh, obviously it's only one specimen from different angles.
Three months sounds very reasonable for that size and quality. Keep it growing, in another three months it can double its mass!
By the way, very nice growing conditions here lately (Holland) and the rest of the week, very constant temperature. Nice weather for crystal growers.
My two big babies are gaining more millimeters and the new layers are perfectly clear 
[Edited on 14-6-2016 by Cucurbit]
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Greets all,
I am so Glad I signed up here the information is just Bloody awesome!, Thank you everybody for your inputs.
I have access to Ammonium Sulfate, and Ferric Sulfate and I want to make some ferric alum to grow crystals. the recipes here will be of immense help.
I have three different bags of the ferric sulfate, one is quite coarse rough looking multiple size tan beads, one is much smaller beads and much more
uniform in size and lighter more yellow color. I believe these are much the same. the last one is marked Ferric sulfate Hydrate and is a fine yellow
powder. Any feedback as to which to use or just info on the Hydrated one, cause I thought ferric could not be a hydrate because of decomposition.??
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
So far is mine solution clear and without any varmint in it. I had to add lil' bit more drops to the solution.
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Varmints in Alum crystal Liquor
Great to hear Neme
I filtered mine again but the stuff floating around came back quicker.
I will have to add some H2SO4 to see if it will help.
Attached is a couple of pics of current crystals that I am growing.
Red one is Potassium ferricyanide
Green is ferrous sulphate
Brown is Potassium sulphate
All are works in progress 
  
[Edited on 23-6-2016 by Dwarvensilver]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Uh, red potassium ferrocyanide? Perhaps you mean ferricyanide? Or does the solution oxidize in air to ferricyanide? Because so far as I can tell,
potassium ferrocyanide should be orangish-yellow, not crimson. No offense to the poster, just curious.
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Ferricyanide
No Offense taken, and you are perfectly right the red one is Ferricyanide not Ferro.
My pardon for the Error and thanks for pointing that out.
Cheers,
|
|
|
Cucurbit
Harmless

Posts: 17
Registered: 19-3-2016
Member Is Offline
Mood: No Mood
|
|
Impressive crystals Dwarvensilver, especially the ferricyanide is very beautiful!
Keep them growing and please keep posting updates 
|
|
|
Chemist_Cup_Noodles
Harmless

Posts: 46
Registered: 27-5-2015
Location: Northern VA
Member Is Offline
Mood: Anticipative
|
|
Quote: Originally posted by Dwarvensilver  | Greets all,
I am so Glad I signed up here the information is just Bloody awesome!, Thank you everybody for your inputs.
I have access to Ammonium Sulfate, and Ferric Sulfate and I want to make some ferric alum to grow crystals. the recipes here will be of immense help.
I have three different bags of the ferric sulfate, one is quite coarse rough looking multiple size tan beads, one is much smaller beads and much more
uniform in size and lighter more yellow color. I believe these are much the same. the last one is marked Ferric sulfate Hydrate and is a fine yellow
powder. Any feedback as to which to use or just info on the Hydrated one, cause I thought ferric could not be a hydrate because of decomposition.??
|
This site certainly is awesome, and I'm glad you're here too! But I see that you are interested in growing some ferric alums, I've actually been
working on making some too for an article on a website run by two other people here on scimad. The one I've been working on producing is Iron (III)
potassium alum. As to your question about the hydrate, I was reading the wikipedia article on the salt, and where it discusses various minerals of the
salt it mentions that there are hydrated forms, albeit quite rare. But for my production of Iron (III) potassium alum, since I lacked any ferrous or
ferric sulfate, I decided to start from the ground up while making it. My procedure was fairly standard for making alums, just tweaked a bit to make
up for my lack of the iron sulfates. Basically, I mix KNO3 and excess sulfuric acid in a flask to produce potassium sulfate and nitric acid
with plenty of acid left over in order to make a highly oxidative mixture. Then, I dissolve iron in slightly excess heated sulfuric acid to form the
ferrous sulfate, and then add that into the flask to get oxidized into ferric sulfate. It's kind of scary though because it evolves a ton of
NO2 but I lack access to strong hydrogen peroxide, so I have to make do. I have yet to try to get some crystals from this, but when I do I
will surely post them!
I'll be honest-- We're throwing science at the wall here to see what sticks. No idea what it'll do.
-Cave Johnson, Portal 2
Add yourself to this map of SciMad members! https://zeemaps.com/map?group=388676&add=1
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Thanks Cucurbit, I will,
Below is a pic of my book shelf at work lol
I work in Research so I have access to literally hundreds of chemicals to try and crystalize.
the big Copper sulphate ones against the wall is what got me started.
Cheers,

|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Ferric potassium sulphate
Wow Chemist_cup_
I am limited as to how much actual chemistry I can do.
I would so love to do the process you outline. Let me know how it goes!
You have given me the bug tho, I have some cobalt film and powder I think I will try to get some cobalt chloride going with a little HCl. 
[Edited on 24-6-2016 by Dwarvensilver]
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|

Well I guess I have to post another few photos of mine 
1st photo: potassium ferricyanide little crystals growing
2st photo: weird effect of potassium ferrocyanide - capillary elevation?
3rd photo: potassium aluminium alum experiment with food dye
4th photo: blue vitriol
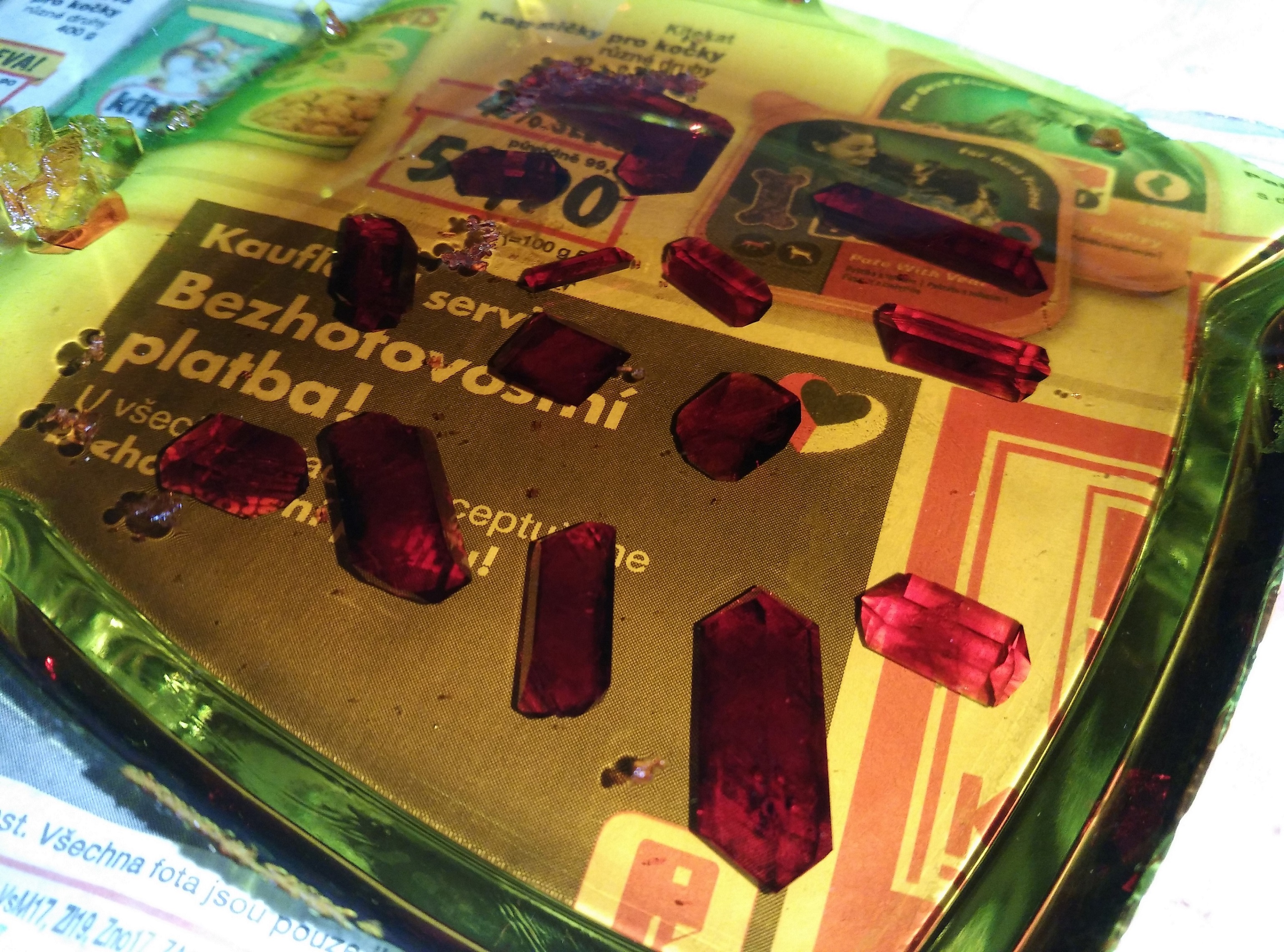   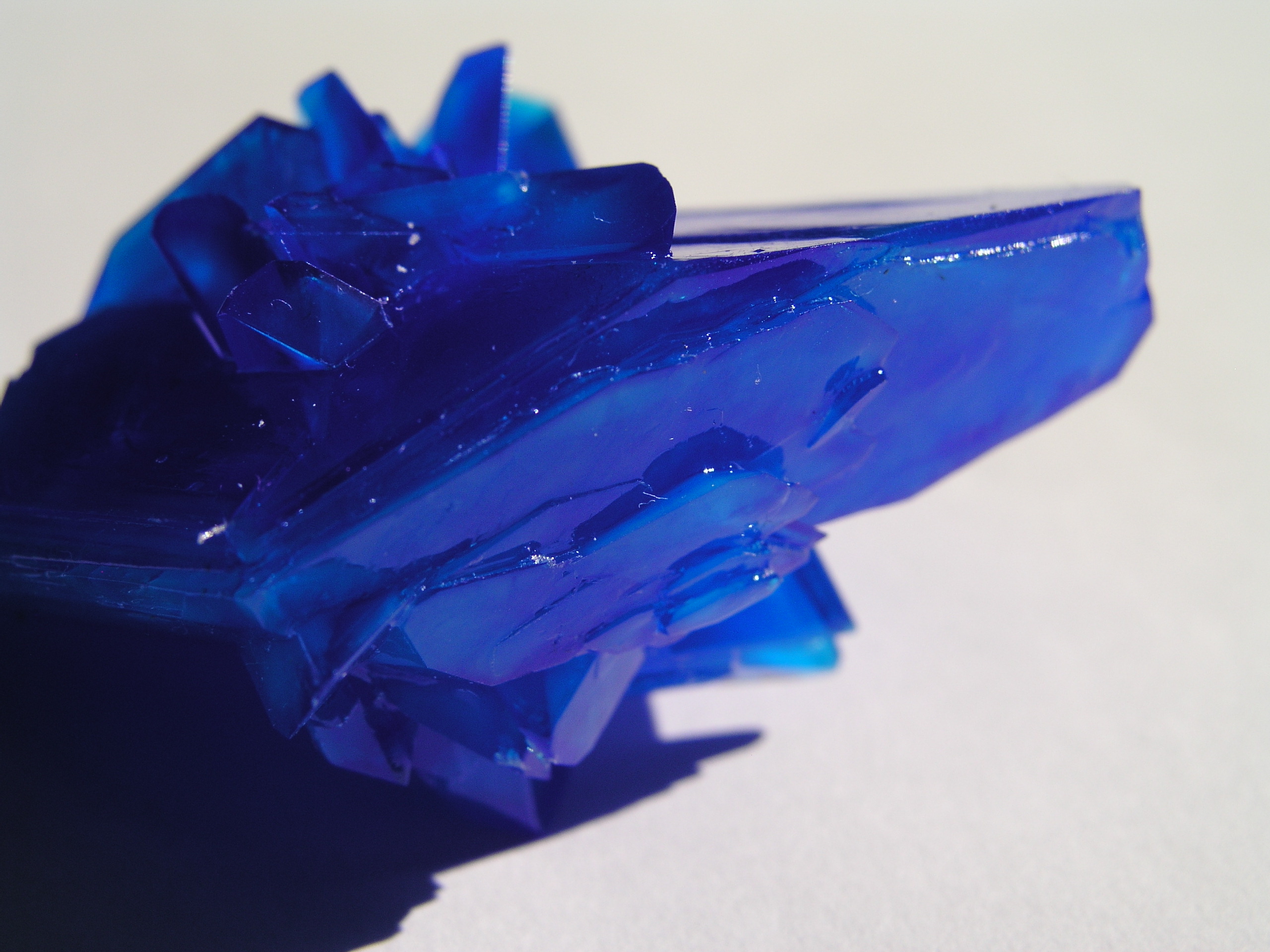
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Nice work Neme. Those look great!!!
I am working on growing Boric acid, (so far it is only making very thin platelets)
Cobalt sulphate, Lead Nitrate (white octocubic shape), I will post pics when they get bigger,
Cheers,
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Quote: Originally posted by Dwarvensilver  | Nice work Neme. Those look great!!!
I am working on growing Boric acid, (so far it is only making very thin platelets)
Cobalt sulphate, Lead Nitrate (white octocubic shape), I will post pics when they get bigger,
Cheers, |
Hello Dwarvensilver, I have also grown boric acid crystals, they were in form of platelets too, but I also noticed some small prismatic crystals,
although Im not sure what conditions are needed to form these prisms, I think they are formed at temperatures ~70 degrees of celsius.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Really pretty stuff. And I'd noticed the same effect in some ferrocyanide solution too. And I think I've finally hit on what I'd like to focus on in
my experiments. Indicators and crystals. 'Pulvers' - I'm bad at. Ochem - bad at. Inorganic chem - can hardly ever get my HCl to do what it's supposed
to because it has so darned much Fe(II) in it. So making double salts might just be it for me, we'll see...
Anyone know of any lead alums?
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
Since lead is usually occuring as II or IV and alums have one I and III, I doubt there is an alum of it.
You still can look for lead tutton's salts but I don't know if they exist either.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by Neme  | Since lead is usually occuring as II or IV and alums have one I and III, I doubt there is an alum of it.
You still can look for lead Tutton's salts but I don't know if they exist either. |
Oh, geesh, I'm bad at chemistry in the late night. I was referring to double salts in general. A lead(IV) double salt would be interesting, as it
might be usefully stable. But I might look around for a lead-something nitrate double salt.
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
I looked through A list of Tutton's salts But no Lead listed in it.
(The Volatile Chemist) I am currently growing a Lead nitrate crystal, What do you think would be good candidates to react with Lead nitrate to make a
double salt?
Cheers,
Dwarven
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Wouldn't it be better to discuss about these salts (ferricyanide, lead nitrate,etc.) In this thread ?: https://www.sciencemadness.org/whisper/viewthread.php?tid=64...
I Don't want to offend anyone, I just want to keep this thread on topic.
|
|
|
| Pages:
1
2
3 |