RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Sodium BiCarb not decomposing as it should at 500-600F
I don't know if I am not doing this properly or if there is something else that needs to be done but I have tried to heat baking soda to 500+ degrees
F and it doesn't seem to decompose into sodium carbonate. I have done this a number of different ways and the only way it has seemed successful was
over a very hot fire where the steel container became orange from the heat. The bicarb seemed to flow almost like a liquid, but it was dry.
As for the 500F test, I had it in the oven and 3 different thermometers verified temps over 500F. I tried leaving it in the oven from up to 12 hours
and it still seemed the same.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quite often there is a lot of NaCl in it as well if bought from a store labelled as 'Bicarbonate of Soda' or 'Baking Soda'.
I found that out when i tried to distill it with sulphuric acid : HCl smell.
Edit:
All you need to do is Heat it, so you're Doing it right, just that the Raw Material is not right.
Suggestion: how about taking this further and arriving at a Working procedure for this kind of Baking Soda ?
NaCl is FAR more soluble in water, so that should be useful.
NaHCO3 = 90g/L, NaCl = 3590g/L
[Edited on 30-4-2016 by aga]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
What do you mean "it still seemed the same"? Anhydrous sodium carbonate has essentially identical physical properties to sodium bicarbonate, both are
free flowing white powders with similar smells. This decomposition is also not exceedingly fast, so it can be difficult to see as it is occurring.
The easiest way to determine if a reaction is occurring is to simply weigh the powder before and after the fact. I'd be surprised if there was no
weight loss at 260°C for 12 hours, that would indicate to me that you don't actually have sodium bicarbonate.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I have not run into this problem, but Arm & Hammer does sell sodium carbonate as "Super Washing Soda." It is tech grade sodium carbonate
monohydrate, really pretty pure.
[Edited on 30-4-2016 by JJay]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by gdflp  | | What do you mean "it still seemed the same"? Anhydrous sodium carbonate has essentially identical physical properties to sodium bicarbonate
|
In my limited experience, the shop-bought (supposedly) sodium bicarbonate is granular, whereas actual sodium carbonate is fine powder.
I have never seen/felt pure sodium bicarbonate.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by aga  | In my limited experience, the shop-bought (supposedly) sodium bicarbonate is granular, whereas actual sodium carbonate is fine powder.
I have never seen/felt pure sodium bicarbonate. |
Huh. In the US, they sell sodium bicarbonate as a free flowing white power(Arm & Hammer Baking Soda), and as I mentioned before it's nearly
indistinguishable from sodium carbonate. I wonder why it's different in Spain.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Arm & Hammer has a very longstanding reputation for quality and purity....
I would be very surprised to see anything misrepresented as sodium bicarbonate since some recipes depend on its tendency to release carbon dioxide
when heated.
Washing it with water can't hurt, except you'll lose some of the bicarbonate. Sodium carbonate is considerably more soluble.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by aga  |
NaCl is FAR more soluble in water, so that should be useful.
NaHCO3 = 90g/L, NaCl = 3590g/L
|
Your number for NaCl solubility is off by a factor of 10, so that might not work as well as you think. As for OP, I want to wait and see how he's
testing his product before we try to determine how to separate a mixture of NaCl and NaHCO<sub>3</sub> when that might be unnecessary.
Aga, care to elaborate why you were distilling what you thought was NaHCO<sub>3</sub> with sulfuric acid? That would just produce massive
amounts of CO<sub>2</sub> with no useful byproduct, or am I missing something?
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Where does the 500F temperature come from, where the conversion just barely starts? That would not be unexpected from a dec. point...CRC says a little
higher 270C. It is really real really easy to use a stove to make soda boil with CO2.
Quote: Originally posted by aga  | In my limited experience, the shop-bought (supposedly) sodium bicarbonate is granular, whereas actual sodium carbonate is fine powder.
I have never seen/felt pure sodium bicarbonate. |
!????????????????????
[Edited on 30-4-2016 by S.C. Wack]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by S.C. Wack  | Where does the 500F temperature come from, where the conversion just barely starts? That would not be unexpected from a dec. point...CRC says a little
higher 270C. It is really real really easy to use a stove to make soda boil with CO2.
|
See this:
http://onlinelibrary.wiley.com/doi/10.1002/ijch.197100086/ab...
| Quote: | Abstract
Thermal analysis of sodium bicarbonate by DTA and DSC showed that the decomposition temperature of this salt is about 100±1°C. A decomposition
temperature of 270°C given in the Handbooks seems to be an error. Adsorbed water is the cause of the small endotherm between 70 and 75°C.
|
270 C sounds much too high to me: it wouldn't be very useful as baking powder.
[Edited on 1-5-2016 by blogfast25]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
You could precipitate sodium bicarbonate out of water with methanol to separate it from sodium chloride. Note that this requires a lot of methanol if
sodium chloride contamination is severe.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Weigh it before and after to get an idea of if/to what extent a conversion is taking place.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
There are several articles and one does give the 270 number...looking some over, it seems they do not completely agree. There may be some general
agreement that the velocity is not so high at 100C. I didn't see anyone guess at a practical industrial temperature and process, for free. How much
air flow in gas and electric ovens? For gas ovens at low temperatures the atmosphere would presumably have some negative effect.
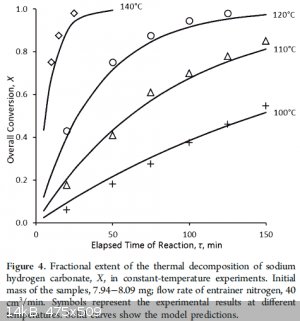
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by gdflp  | | Aga, care to elaborate why you were distilling what you thought was NaHCO<sub>3</sub> with sulfuric acid? |
I really cannot recall exactly.
Probably i was simply neutralising some sulphuric with what says 'sodium bicarbonate' on the label and noticed a strong whiff of HCl.
This stuff is granular and, same as the OP, appears to do nothing when heated, although CO2 is evolved (puts out a flame).
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
I'm wondering what hydrate the Sodium Carbonate would be after heating? I have heated Na2CO3 decahydrate on the stove until complete evaporation to
get what I believed was anhydrous and done the same by placeing moist/damp decahydrate crystals in the oven on high (500-550F) and getting a similar
looking "crystal" as the anhydrous from the stove top. In each case the decahydrate turned to a liquid form and slowly boiled away.
I would suspect the bi-carb -> carb would leave anhydrous or possibly monohydrate (getting the water molecule from the conversion process??)
I did a test of heated bi-carb, regular bi-carb and carbonate (monohydrate), I prepared 25ml of DH2O and to each I added 10g of each of the
carbonates. The unheated bi-carb produced no heat and eventually settled with a thick layer of "powder" on the bottom. The other two both produced
about the same amount of heat. The conveted bicarb->carb dissolved quickly with no signs of remaining powder (although it was very fine/powdery
and solution was cloudy) and the carbonate monohydrate didn't fully dissolve although I thought it should have with a 50g/100ml @20C solubility (the
grains were MUCH larger than the baking soda convert). I then added another 8g of the converted bi-carb->carb powder and it seemed to not dissolve
into the solution and settled like the undissolved bi-carb powder.
So it seems that there has been at least some conversion, possibly a very high percentage. I am wondering if heating on a stove-top would be a better
option than heating in the oven. I know when I heated over intense flame (forced air forge) I think I heated past the decomp state of Carbonate as I
have some very hard chunks of a yellow crystaline substance which I suspect may be Na2O? The thing is that Na2O is supposed to react violently with
H2O to form NaOH buy my yellow crystals did not and took 4-5 hours to dissolve a 1g chunk in about 20ml of H2O...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Wiki says the decompostion of bicarb starts at 50 C, and all info i ever found says you get CO2 plus steam if heated in a pan on a stove,
eventually leaving a dry powder of sodum carbonate.
S'not what you or i got.
My raw material is a 1kg bag of store-bought stuff that says 'bicarbonato de sodio' on it.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
OK, having performed this at least twice, the correct procedure is to stir a frying pan containing dry powdered NaHCO3. When the pan is hot enough,
the fine powder produces bubbles.
The essential problem (or benefit for fire suppression purposes) is that to my surprise the NaHCO3 powder that is not in direct contact with the pan,
is not even hot to the touch! Latter on, as it is converted to Na2CO3, the salt retains heat better and placing the product in a plastic container
immediately is not advised.
Your experiment highlights this heat insulation quality of NaHCO3's endothermic decomposition, which together with the formation of CO2, is probably
why it is good for fire suppression.
[Edit] Here is a comment from a typical source (link: https://www.google.com/url?sa=t&source=web&rct=j&... ), to quote:
"NaHCO3 is a solid particle powder whose overall kinetics of decomposition are relatively well studied. The decomposition of NaHCO3 was
represented in the kinetic model by the overall chemical process [7, 16].
2 NaHCO3 (s)→ Na2CO3(s) + H2O + CO2 (reaction (1) was corrected by me)
Na2CO3 (s)→ Na2O + CO2 (2)
It should be noted that the use of an overall NaHCO3 decomposition process in the kinetic model is valid for small sized particles (less
than 10 to 20 microns), which will completely evaporate in the flame reaction zone. The decomposition process begins with sodium carbonate
melting at 270 °C (543.15 K) and proceeding to form CO2, H2O, and Na2CO3(s). The second step, decomposition of Na2CO3(s), produces Na2O
and CO2. The overall decomposition process is endothermic; enthalpy change is 135 kJ/mol and when above 400 K, the decomposition is very fast.
The decomposition reaction rate demonstrates a first order dependence based on the amount of unreacted NaHCO3 (A=1.43E11, 1/s; E=102kJ/mol;
373−473K [16]). The gas-phase suppression kinetics for NaHCO3 can be represented by reactions forming NaOH, NaO and Na2O species. In the
presence of moisture NaHCO3 hydrolysis also occurs, relatively fast, through the following two reactions
NaHCO3 + H2O = NaOH + H2CO3 (3)
H2CO3 = H2O + CO2 (4) "
-------------------------------------
I have just decided to design a homemade fire extinguisher with vinegar and salt (to limit the hydrolysis of the sodium bicarbonate) that would
combine a large excess of dry NaHCO3 with a CO2 gas propellant.
[Edited on 9-6-2016 by AJKOER]
|
|
|