franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Primary Amine metathesis
It is said that if the only tool you have is a hammer , you tend to see every
problem as a nail. Conventional solution chemistry in ambient conditions requires
following reaction pathways over many counterintuitive steps with progressively
lower yield , but forming oxidating groups directly with gas phase reagents may
often be done in one step.
Reviewing known reactions of primary amines we see that:
1 )
amine + nitrous oxide -> azide
H2N- + N2O -> H20 + -N3
2 )
amine + ozone -> nitro
H2N- + O3 -> H2O + -NO2
3 )
amine + halogen -> di(halo)amine
H2N- + 2 F2 -> 2 HF + -NF2
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Azides from nitrous oxide
1 )
Cyanamide may react with nitrous oxide to form Azidocyanate:
H2N-CN + N2O -> H2O + N3-CN
http://www.sciencemadness.org/talk/viewthread.php?tid=925#pi...
Hydrazine should react with nitrous oxide to form : ? ( at cold temperature )
NH2.NH2 + 2 N2O -> 2H2O + N6 . . . ( a string of six nitrogens )
an N5 radical is known _
http://www.afrlhorizons.com/Briefs/Dec01/PR0106.html
Here is a very interesting read and an outline of approaches on this ,
- check out the Antimony azide structure ,
http://stinet.dtic.mil/cgi-bin/GetTRDoc?AD=ADA422668&Loc...
As in this related thread
http://www.sciencemadness.org/talk/viewthread.php?tid=6027 N4 !?
the possibility here is if N6 can be further tautomerized under pressure
[Edited on 4-10-2006 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Boron explosives
http://www.sciencemadness.org/talk/viewthread.php?tid=1244#p...
Dahl, G.H. & Schaeffer, R. J. Am. Chem. Soc.1961, 83, 3032
Borazine hydrochloride + Sodium tetrahydroborate -> Cyclotriborazane
2 B3N3H6.3HCl + 6 NaBH4 -> 2 B3N3H12 + 6 NaCl + 3 B2H6
http://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/33098_...
( page 539 )
http://www.fsec.ucf.edu/hydrogen/research/pdf/FY02_Technoeco...
( page 9 , also has excellent bibliographic references )

http://www.sciencemadness.org/talk/viewthread.php?action=att...
Observing the structure of Tri-aminoborane it is immediately apparent each ring
atom has 4 bonds, indicating that this is the cyclic trimer of Aminoborane joined
by 3 coordination bonds.
Reacting this with Nitrous Oxide should yield Azidodiazoboron
3(:NH2-BH2 ) + 6 N2O -> 6 H2O + 3 N3-BN2
N3-BN2 -> BN +2 N2
The question pending is weather the oxygen from N2O will instead bond to boron
or not.
http://www.hydrogen.energy.gov/pdfs/review06/bes_st2_autrey....
http://www.iphe.net/Storage%20-%20Lucca/posters_PDFs/CH1%20A...
[Edited on 27-9-2006 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Nitro from Ozone
2 )
Hydroxylamine reacts with ozone to form nitric acid:
H2NOH + O3 -> H2O + HNO3 . . . ( curious but not useful )
Cyanamide may react with ozone to form Nitrocyanate:
H2N-CN + O3 -> H2O + NO2-CN . . .
http://www.sciencemadness.org/talk/viewthread.php?tid=925
this could serve as a plasticizer for hexanitrobenzene or octanitrocubane
just as teranitromethane does for trinitrotoluene and trinitrobenzene
 
Cyanamide above here on the left forms into the cyclic trimer Melamine
( Triaminotriazine ) seen on the right. Just as it's monomer above does ,
this may react with ozone to form Trinitrotriazine.
(=N-C(NH2)=)3 + 3 O3 -> 3H2O + (=N-C(NO2)=)3

Trinitromelamine discussed here
http://www.sciencemadness.org/talk/viewthread.php?tid=173#pi...
Ozonides
http://www.sciencemadness.org/talk/viewthread.php?tid=6342#p...
Benzene Triozonide
http://www.sciencemadness.org/talk/viewthread.php?tid=1181#p...
Despite their straight forward application , obtaining or producing nitrous oxide
or ozone is the chief obstacle to their use , unless something like the plasmoid
production of nitric oxide can be devised.
http://www.sciencemadness.org/talk/viewthread.php?tid=4092#p...
How Nitrous Oxide may be prepared http://en.wikipedia.org/wiki/Nitrous_oxide
Ozone is produced by high voltage applied between two coaxially arranged
electrodes, creating a corona discharge inside a quartz glass tube. The electrodes
are separated from each other by a dielectric and two discharge chambers,
through which gas flows.
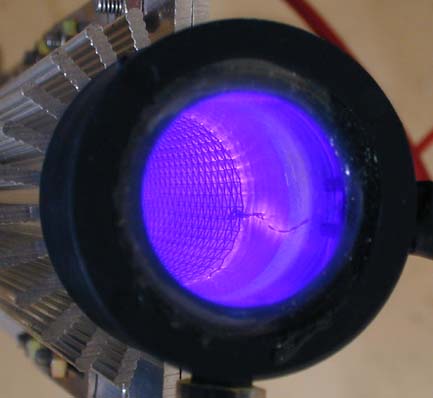
Some of the oxygen molecules in the input gas break down in the electric field
and immediately attach themselves to free oxygen molecules, forming ozone.
Efficieny is perhaps 10% since ionizing the oxygen or air imput also heats it,
thus to prevent heat disassociation of the ozone created, supplemental cooling
is required. Small units are air cooled, larger outputs require water cooling.
All industrial-scale ozone generators make use of this method. Because ozone is
very reactive, it is also unstable. It cannot therefore be stored and has to be
produced where and when it is needed.
Worldwide less than 300 million dollars annually is spent on ozonation machines
so there are few manufacturers of commercially available units, and these are
mostly home air and water purifiers of low output less than 2 grams per hour.
The output can be increased by 5 times just by using a membrane type
" oxygen generator " or concentrator to separate oxygen from air. Ozone is
produced in concentration from 6 to 12 percent of the processed oxygen.
Units that integrate both oxygen generation as a feedstock for the ozonizer are
very few, requiring their separate purchase and flow matching. Typical costs for
just an ozonizer is from a few hundred to several thousand dollars. Consider to
spend about 100 dollars per gram of hourly output. Power required is around
150 watts per 10 grams of output per hour..
Clearly not all reactions result in metastable products. Hydrazine is completely
oxidized by ozone. Hydrogen peroxide will interact with ozone to form Trioxidane.
http://en.wikipedia.org/wiki/Trioxidane

It's fleeting existence may however be rendered somewhat more stable if it is
allowed to form trioxidane in the solid phase somewhat like chlorine absorbed by
caustic soda to form hypochlorite.
I propose that Sodium Percarbonate may form a metastable Trioxidane compound
by reacting with ozone.
Urea is also oxidized by ozone so it's peroxide unfortunately cannot serve the
same purpose.
(NH2)2CO + O3 -> N2 + CO2 + 2 H2O
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Di(halo)amines from halogens
3 )
Halogens will replace hydrogen attached to nitrogen
Here are current applications of Fluoro amines
http://www.pat2pdf.org/patents/pat6395899b1.pdf
http://www.pat2pdf.org/patents/pat6417355b1.pdf
Hexachloromelamine
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1705...
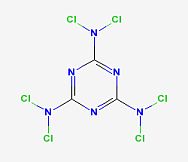
This appears to be an explosive given the dichloramine groups.
A related thread is here _
http://www.sciencemadness.org/talk/viewthread.php?tid=2945#p...
Pentafluoroguanidine
http://webbook.nist.gov/cgi/cbook.cgi?ID=C10051066&Units...
FN:C(NF2)2

Is a liquid , this , and several of its adducts with alcohols, are shatteringly
powerful explosives, if I have correctly analysed its thermodynamic potential
2CNF(NF2)2 -> 2CF4 + 3N2 + F2
3300 kcal/kg . . . in a word , W O W !
While a molecular Boro Fluoramine compound is conspicuosly absent at present
this does not preclude a binary composition. Boron yields enormous energy.
4B + 3O2 -> 2B2O3
4300 kcal/kg
It seems reasonable that Pentafluoroguanidine may dissolve Diborane B2H6
http://webbook.nist.gov/cgi/cbook.cgi?ID=C19287457&Units...
FN:C(NF2)2*B2H6 -> BF3 + BF2CN +N2 + 3H2
This of course will likely be a sensitive primary explosive , but does serve
to demonstrate the unexploited potential with such compounds.
.
[Edited on 27-9-2006 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Derivatives of Guanidine
Aminoguanidine tautomers
 
DiAminoguanidine tautomers
  
These possess many nitrogen bonded hydrogens which can be exploited with
any of the above cited chemistry to form Azides by the action of Nitrous oxide ,
Nitroamines by the action of Ozone , or Haloamines by the action of halogens.
[Edited on 6-11-2007 by franklyn]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
hexachloro melamine... interesting idea. Is melamine available in its unpolymerised state?
Let's not forget however that trinitromelamine is extremely unstable and hydrolyses quickly. I wonder how the monomer, nitrocyanide NO2CN could be
made with methods other than O3.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
Is melamine available in its unpolymerised state? |
http://www.chemicalland21.com/industrialchem/organic/CYANAMI...
Dormex is a 50% aqeous solution of hydrogen cyanamide
distributed by the german firm Degussa
There looks to be a tautomer of the form HN=C=NH
http://www.chemthes.com/entity_datapage.php?id=2143
http://www.chemicalland21.com/industrialchem/organic/MELAMIN...
Dicyanamide is available from
http://www.industrial-chemical-manufacturer.com/industrial-c...
Melamine is discussed here _
http://www.chemthes.com/entity_datapage.php?id=2145
Melamine, a plastic (used for telephones), was originally manufactured from
calcium cyanamide by electrolysis, but this process was obsolete by 1960.
While calcium cyanamide continues to be used as an agricultural fertilizer,
the last hydrogen cyanamide plant in the western world closed in 1971.
It is now produced from urea (with ammonia) at high temperature and
pressure which pyrolyzes to form melamine.
While I'm aware of cyanamide as an agricultural chemical and staple industrial
precursor apparantly it has some handling concerns. It is caustic and toxic ,
while the solid is stable, concentrated aqueous solutions of cyanamide may
undergo explosive polymerization.
Incompatibilities: Contact of cyanamide with water, acids, or alkalies may
cause a violent reaction at temperatures above 40 degrees C (104 degrees F).
Mixture of cyanamide with 1,2-phenylenediamine salt may cause explosive
polymerization.
http://www.osha.gov/SLTC/healthguidelines/cyanamide/recognit...
.Cyanamide is highly soluble in water, aliphatic alcohols, ethers, esters and amides.
It is sparingly soluble in chlorinated or aromatic hydrocarbons, unsoluble in aliphatic
or cycloaliphatic hydrocarbons. Ketones cannot be used because they can react
with cyanamide under certain circumstances. Also called Urea anhydride, in acidic
solutions cyanamide adds water forming urea, releasing the reaction enthalpy of
-2582 kJ/kg.
As is indicated here _
obtaining hydrogen cyanamide from calcium cyanamide
is done in one simple step
http://www.cyanamide.com/content/production.htm
http://www.cyanamide.com/content/reactions.htm
http://www.cyanamide.com/content/reactions_1.htm
http://www.cyanamide.com/content/reactions_2.htm
http://www.cyanamide.com/content/reactions_3.htm
[Edited on 28-9-2006 by franklyn]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well, these links you provided probably point to sellers targeting businesses and industry, rather than private individuals.
Interesting that melamine can be made from urea (attachement):
| Quote: | Melamine is made industrially by cyclising urea. The carbon dioxide and ammonia are reconverted into urea.
Silica/alumina catalyst
Alan Heaton (Ed.) An Introduction to Industrial Chemistry, Blackie (1996) |
Would be nice to find the detailed conditions, or also how to depolymerise thusly produced melamine back to cyanamide...
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
I wonder how the monomer, nitrocyanide NO2CN could be made with methods other than O3. |
Considering alternative methods and the actual expense with the use of nitric acid
and reagent chemistry , its a wonder why ozone has not been exploited for small
scale production of nitro compounds where it has real economic competiveness.
The only objection may be to the relatively long period of synthesis subject to the
rate of ozone production. Unlike electrical means employed for direct synthesis of
nitrogen dioxide from air , ozonation is a breeze. The preparation of nitric acid by
the Birkeland-Eyde process requires that Nitrogen and Oxygen in air are combined
to produce nitric oxide at a temperature of 3000 degrees Celsius. The heat for
disassociation and reformation is provided by electric arc at the rate of about
15 megawatt hours per ton, which is very expensive. This is more than for the
production of Aluminum at 14 megawatt hours per ton.
I quote _
The apparatus which effects this transformation is essentially a gigantic arc light
in a chimney through which a current of hot air is blown. The more thoroughly the air
comes under the action of the electric arc the more molecules of nitrogen and oxygen
will be broken up and rearranged, but on the other hand if the mixture of gases remains
in the path of the discharge the NO molecules are also broken up and go back into their
original form of NN and OO. So the object is to spreadout the electric arc as widely as
possible and then run the air throughit rapidly. In the Schönherr process the electric arc
is a spiral flame twenty-three feet long through which the air streams with a vortex
motion. In the Birkeland-Eyde furnace there is a series of semi-circular arcs spread out
by the repellent force of a powerful electric magnet in a flaming disc seven feet in
diameter with a temperature of 6300° F. In the Pauling furnace the electrodes between
which the current strikes are two cast iron tubes curving upward and outward like the
horns of a Texas steer and cooled by a stream of water passing through them. These
electric furnaces produce two or three ounces of nitric acid for each kilowatt-hour of
current consumed.
In the more recent Wisconsin process which had been operated on the pilot plant scale,
atmospheric oxygen, and nitrogen are combined in a high temperature regenerative
furnace operating at about 2000 C. Nitric oxide is formed with a yield of nearly 2%. The
process has proved technically feasible but is not in commercial operation as presumably
it cannot compete economically with the ammonia oxidation route. The third method of
directly combining oxygen and nitrogen is the nuclear nitrogen fixation route. Yields of
nitrogen oxide of 5-15% have been reported by exposing air at 150 psi and 400 F to
radiation from uranium 235. The attractiveness of this process will ultimately depend
on the efficiency of conversion of the nuclear radiation into chemical energy and also
on the cost of the radiation. _ close quote ( my comment , JEEEZ )
Ozone as it has been made historically
http://www.lateralscience.co.uk/marum/index.html
Ozone as it is made in practice
http://www.lenntech.com/ozone/ozone-generation.htm
Excellent forum thread on home made ozonizer here _
http://www.sciencemadness.org/talk/viewthread.php?tid=1774#p...
Preparation of nitric oxide
http://www.fas.harvard.edu/~scdiroff/lds/Geophysics/SmoginaB...
http://kronjaeger.com/hv/hv/exp/no/index.html
http://www.1911encyclopedia.org/Nitrogen
Birkeland Eyde Process See pg 8 ( takes a moment to download, be patient )
http://assets.cambridge.org//052178/2848/excerpt/0521782848_...
Home made nitrous oxide ( three pages , links on this page )
http://blog.modernmechanix.com/2006/03/07/the-gas-that-makes...
Lateral Investigations into -NO synthesis ( no one has yet cried Eureka )
https://sciencemadness.org/talk/viewthread.php?tid=1013
[Edited on 28-9-2006 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
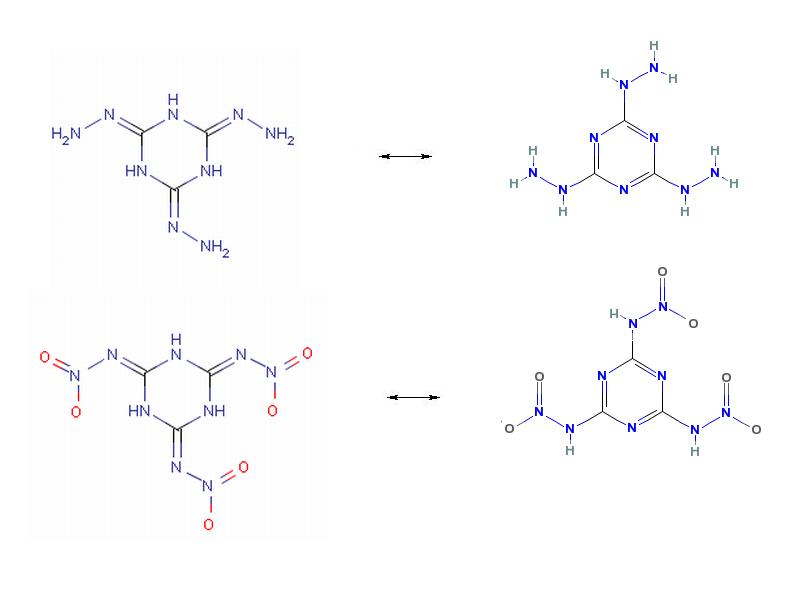
Ozonolysis of Cyanuric Trihydrazide
Cyanuric Trihydrazide also known as Trihydrazinotriazine occurs in two tautomeric forms
which are the first top two structures shown below.
References _
http://chem.sis.nlm.nih.gov/chemidplus/ProxyServlet?objectHa...
http://webbook.nist.gov/cgi/cbook.cgi?ID=C10105427&Units...
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6627...
A minor mention of it here _
http://www.sciencemadness.org/talk/viewthread.php?tid=4094#p...
Treatment of this with ozone should yield Cyanuric Trinitroamine which similarly must occur
in two tautomeric forms each seen below it's precursor above.
It has not escaped my attention that the apparent acidity can be
exploited to form salts.
From what I can find this is not a known compound.
Hexahydrotrinitotriazine shown separately below is the nearest similar structure.
[Edited on 16-2-2008 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Hexahydrotrinitotriazine shown here is the nearest similar structure.
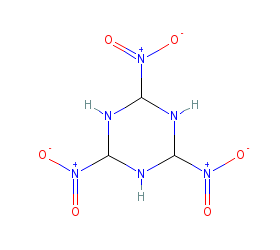
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I was sorting out some of my old notes to throw away as much as possible and I stumbled upon this note on N-chloromelamines. I remembered this was
talked about somewhere so I typed it out:
Chemical Abstracts, 60 (1964) 2988h: Chloromelamines were prepared by chlorinating melamine in water. Required was 20-100 parts of water, the
quantity of melamine great enough so that the final c(HCl)<1N. Cl2 was passed into vigorously stired solution of 6,3 parts of melamine in 350 parts
water for 30 min to yield 14,6 parts hexachloromelamine, m.p. 148-150°C. Similarly prepared was 90% trichloromelamine, dec. 175°C.
I didn't include the original reference, but from the abstract it sounds like it was some patent.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Caged and polymer Triazine (sym)dinitrohydrazides
Not another flight of fancy you say 
It occurred to me that the charge neutral Nitrogen trioxide free radical could be
applied to the oxidation of primary amines and adding there a nitro functional group.
Unlike ozone which replaces the two hydrogens of the amine with two of oxygen ,
oxidation by the O2N=O radical attaches a nitro functional group forming a nitroamine
but additionally leaves a remaining free bond on the amine.

This secondary free radical will immediately bond to another like it to form a composite
with these two nitroamines , a symetrical dinitrohydrazo functional group.
Because of its fleeting existence ( two moles of NO3 combine to form one of O2
and two of NO2 ) it's a major challenge to both produce NO3 in situo and avail it
of intimate proximity with the intended reducing agent. An intriquing solution to
this was originated many years ago and is cited in COPAE on pages 228 and 229 ,
which is available in the forum library and is also discussed here _
http://www.sciencemadness.org/talk/viewthread.php?tid=6279
and here _
http://www.sciencemadness.org/talk/viewthread.php?tid=1716#p...
I quote :
" A platinum gauze anode is used. It is immersed in an acetone solution of calcium
nitrate which is kept continuously saturated with ethylene which is bubbled through
in such manner that it sweeps over the surface of the platinum gauze. "
U P D A T E
I understand the use of acetone as a non-participatory solvent is needed to obviate
the formation of nitric acid as well as be miscible with the other organics. One cannot
evade the presence of water since it is a principal product in this supposed proceedure
in which I propose ammonium nitrate be subjected to electrolysis to provide the nitrate
ion precursors for NO3 radical formation. One can nevertheless greatly minimize such
exposure by partitioning with two solvents as I describe below.
A deep glass pan will be needed on the bottom of which will be placed the anode ( + ).
This can be platinum foil if you fancy or a sintered graphite automotive gasket for
expedience and thrift. This is to be connected to a wire of teflon insulation with a strip
of foil then heavily covered with epoxy to protect these metals from corrosion. Situated
above this horizontally on some glass struts will be the cathode ( - ) comprised of
stainless steel wire mesh similarly connected to the power source. The entire assembly
is to be submerged in the pan.
Prepare a solution of melamine by adding this to hot water in the pan until saturated.
To this then add the ammonium nitrate which being extremely soluble will displace the
melamine which precipitates on top of the anode on the bottom. To this then one can
introduce a second solvent which is denser than water and immiscible with it, what
water is formed can be segregated into the upper aqueous phase to obviate hydrolysis
reactions. Dichloromethane sparingly miscible in water < 1.3% fulfills this need quite
well and may be introduced through a pipet last just before proceeding with electrolysis.
Another question is will it inhibit or present a barrier to anion permeability. A further
practicle consideration is that temperature should be kept as low as possible , near
to freezing to conserve the volatile dichloromethane. Placement of the pan within
another larger pan containing salted ice will serve the purpose . For comparison ,
ozonolysis is usually done in dichloromethane at the temperature of dry ice also to
minimize decomposition of the ozonides produced.
Now turn on the power.
The reaction looks to be straight forward. NH4 cations migrate to the cathode above
to release hydrogen and ammonia which can be vented or fed through a fume hood
( polyethylene bag taped around the rim of the pan ) to a fountain for collection.
The hydrogen can safely be flared from there.
NO3 nitrate anions will migrate down through the precipitated melamine and have
their charge neutralized at which point this supposed synthesis will take place. What
water is produced that transiently forms nitric acid will itself be electrolyzed into
Hydronium cations ( thereby be purged upward ) and reconstitute the nitrate anion.
In figure 1 is an Argus lab ( http://www.arguslab.com ) depiction of the
trinitroamino triazine ( melamine ) radical to be formed.
What happens next is the point of this speculation.
 Figure 1 Figure 1
C L I C K F O R F U L L S I Z E ( Then click that image again )
http://img156.imagevenue.com/img.php?image=07978_Trinitroami...
Tetrahedral hexa(sym)dinitrohydrazo tetratriazine ( Figure 2 )
Structurally analogous to Hexamethylenetetramine in which instead of six methylenes
there is are six dinitrohydrazo groups joining four triazine rings instead of single amines.
 Figure 2 Figure 2
C L I C K F O R F U L L S I Z E ( Then click that image again )
http://img137.imagevenue.com/view.php?image=08787_Tetrahedra...
Octahedral Deca(sym)dinitrohydrazo octatriazine ( Figure 3 )
With eight triazine rings it is much larger and for this reason less likely to form in any
meaningful quantity.
 Figure 3 Figure 3
C L I C K F O R F U L L S I Z E ( Then click that image again )
http://img150.imagevenue.com/img.php?image=09166_Octahedral_...
A cubic form is not possible , this would require four way bonding. Larger three
way bonded platonic or geodesic variants are likely only imaginable. All of these
can occur in one of two chiral enantiomers.
Because it is less strained than discrete molecules the most realistic result is the
polymer formation shown here _
 Figure 4 Figure 4
C L I C K F O R F U L L S I Z E ( Then click that image again )
http://img107.imagevenue.com/view.php?image=09284_PolyTrinit...
The tendancy of a molecular sheet to warp and roll into a cylinder shape suggests
that tubes analogous to carbon nanotubes may be formed.
Unstructured amorphous polymer without defined plane formation can also result.
One final thought on this for the discrete tetrahedral molecule is that an ammonium
ion might serve to coordinate its formation and in this process become enclosed and
encapsulated within the surrounding cage. There is enough room within for a small
molecule and the nitrogen ring atoms at each apex provide a preferential locus for
the hydrogens. Similar to cations in which crown ether encloses a positive ion, the
enclosed ammonium provides a net postive charge to the whole molecule which then
will migrate up to the cathode to neutralization its charge. What can result is that
you end up with a neutral molecule containing an ammonia atom and what ?
mono-atomic hydrogen ? But only if that is not able to escape or bond to its host.

C L I C K F O R F U L L S I Z E ( Then click that image again )
http://img24.imagevenue.com/img.php?image=49159_ammonium_Tet...
Thank you for your time and I welcome your derision.
The attached *.zip file contains the Argus lab files of the molecules depicted if you
care to investigate this further.
.
Attachment: Trinitroaminotriazine radical.zip (54kB)
This file has been downloaded 905 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
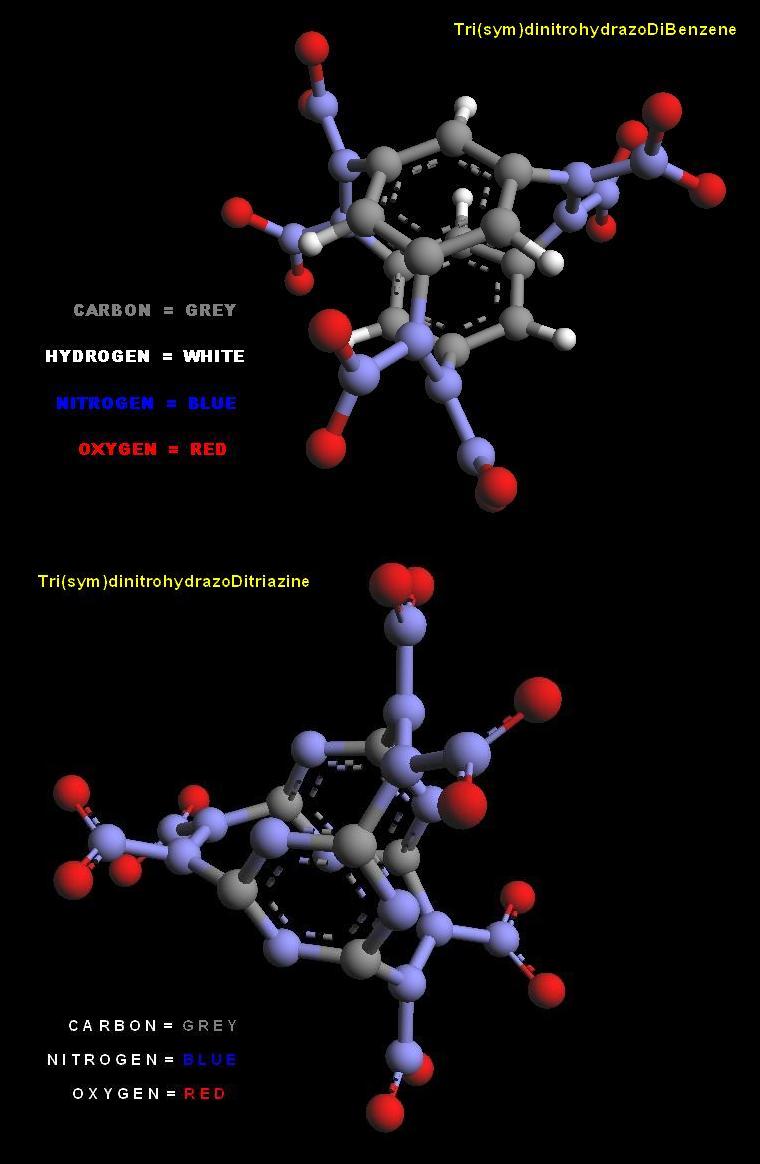
Tri-dinitrourea Di-triaminobenzene
U P D A T E
[img]http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=66952[/img]
It occurs to me that Triaminobenzene can be substituted for Melamine in the
application disscussed above. Although somewhat strained much simpler
molecules may be formed and with Melamine too.
Argus lab files are in this zip file _
[Edited on 12-8-2007 by franklyn]
Attachment: Tri(sym)dinitrohydrazoDibenzene.zip (12kB)
This file has been downloaded 937 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
U P D A T E
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
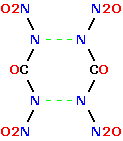
Bi(sym) Dinitrourea
In the same setup as above here , subjecting an aqueous mix of Urea
and Ammonium Nitrate to electrolysis should form and condense two
mols Dinitrourea so _
2 [ H2N.CO.NH2 + 2 ONO2 -> 2 H2O + (-NNO2 )2:CO ] -> ( O2NN-NNO2 )2(CO)2
forming Bi(sym) Dinitrourea pictured here _
Related structures _
http://www.sciencemadness.org/talk/viewthread.php?tid=6042#p...
.
|
|
|