wish i had a kraken!!!
Hazard to Others
  
Posts: 157
Registered: 22-3-2012
Member Is Offline
Mood: No Mood
|
|
Antimicrobial Copper Coatings failure
Well, actually I had an idea on how to make inexpensive antimicrobial filters and these photos refer to that idea. but I failed !
If you have any idea on why I failed to achive my goal, I'd be more than happy to hear it :-)
I have explained my idea on youtube link below:
https://www.youtube.com/watch?v=fuE-xGX9cFk
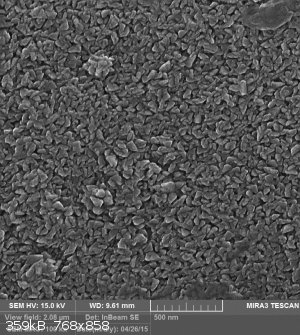 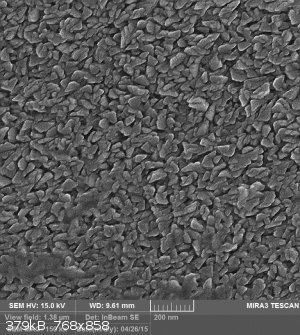
[Edited on 10-8-2016 by wish i had a kraken!!!]
[Edited on 10-8-2016 by wish i had a kraken!!!]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
My limited understanding on how the antimicrobial mechanism works on zeolite structures, for example, is that it permits a cycling of copper through
valence states (including Cu(0), Cu(l), Cu(ll) ). In the presence of O2, this transition metal valence change can form reactive oxygen species (ROS
include the likes of the superoxide radical anion, hydroxyl radical, H2O2..). It is the latter ROS that are primarily and continuingly responsible
for disinfecting.
Note, aqueous Cupric salts, like CuSO4 have some toxic properties with respect to bacteria, fungi and algae. As such CuSO4 is directly added at times
to swimming pools at low levels as high levels can produce acute pain and poisoning in humans as well.
Here is a not particularly revealing source (as pages are omitted) available at
https://books.google.com/books?id=vWWgPhzWUTwC&pg=PA1673&lpg=PA1673&dq=Cu+%2B+Cu(ll)+%3D+2+Cu(l)&source=bl&ots=MNEFwqLVHO&sig=
YHyk_F1_iOxHfQq5LDEnji4yjBU&hl=en&sa=X&ved=0ahUKEwily-_bnrfOAhWsCMAKHareAJQQ6AEIUTAH#v=onepage&q=Cu%20%2B%20Cu(ll)%20%3D%202%20Cu(l)&a
mp;f=false
I strongly advise you do not drink your copper enriched water. My experience with having just allowing spring water to sit in a copper cup for less
than a minute and then consuming has led me to stop this practice (powerful and potentially sickening brew depending on the water's pH, CO2
content,...that aid in the dissolution of the copper oxides coating).
[Edited on 10-8-2016 by AJKOER]
|
|
|
|