Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Help with 2,2'-bisthiazole
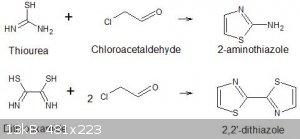
I am trying to prepare 2,2'-bisthiazole or 2,2'-dithiazole and I have a problem.
I am using a modified version of the reactions described in Vogel's Textbook of organic chemistry 3rd Ed p840-841 where he describes the preparation
of 2-aminothiazole and 2-amino-4-methylthiazoles by the condensation of 2-chloroacetaldehyde or chloroacetone with thiourea. The chloroacetaldehyde is
generated in situ from 1,2-dichloroethyl-ethylether. I do not have any of the latter compound but I do have some chloroacetaldehyde dimethyl acetal
which should yield the chloroacetaldehyde in situ in a similar fashion. To test this I ran the 2-aminothiazole synthesis as per Vogel but substituted
the 1,2-dichloroethyl ethyl ether with chloroacetaldehyde acetal in a molar equivalence. The reaction was completely successful giving a similar yield
to the original procedure. The reaction time was reduced from about 2 hours to about 25 minute.
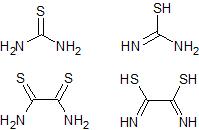
The comparison between thiourea and dithiooxamide thioamide and enol forms
The reactive species in this reaction is probably the enolized thioamide and several examples of this type of reaction are known. It appears that in
thiourea the concentration of the tautomeric amino-formothiolimine is sufficient to bring about the react. In my modification of the reaction I am
trying to use dithiooxamide which is the diamide of thiooxalic acid or basically two thioamide groups back to back. It acts as an acid by virtue of
the partial enolization of the thioamide groups to a similar formothiolimine group. So dithiooxamide (rubeanic acid so called) should react with two
molar equivalences of chloroacetaldehyde to give the required 2,2'-bisthiazole.
C<sub>2</sub>N<sub>2</sub>S<sub>2</sub>H<sub>4</sub> +
2C<sub>4</sub>H<sub>9</sub>O<sub>2</sub>Cl →
C<sub>6</sub>H<sub>4</sub>S<sub>2</sub>N<sub>2</sub> + 2HCl + 4CH<sub>4</sub>O
I tried refluxing 3g of dithiooxamide with 6.3g chloroacetaldehyde dimethyl acetal in 30ml of methanol, most of the dithiooxamide dissolves but even
after hours of refluxing simply crystallises out again on cooling. I am wondering if I might be able to add a catalysts. A base might encourage
enolization of the dithiooxamide but this compound is not stable towards strong bases such as sodium hydroxide or an acid to accelarate the hydrolysis
of the acetal?
Does anyone have any experience of similar reaction or have any ideas/suggestions regarding possible catalysts or other modifications to the
conditions?
[Edited on 11-8-2016 by Boffis]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Chloroacetaldehyde, huh? Nasty looking thing. Anyway TsOH cleaves acetals, giving aldehydes + TsOMe.
You could see success using a base such as DBU to make the chloroacetal react with the deprotonated iminothiol, then use TsOH to cleave the acetal and
provoke cyclization.
But recall that haloethers are very reactive compounds. It's possible that 1,2-dichloro-3-oxapentane actually reacts with the amine residue at the
2-position and this hemiacetal eliminates ethanol to the imine. Acetals do not cleave quite as easily, and won't react with most bases. I assume
chloroacetaldehyde polymerizes or is otherwise very unpleasant to work with.
Another idea is to use glycolaldehyde phenyl (or mesityl) ether. Phenyl ethers react with thiolates, after all, and this compound should not
polymerize. A catalyst is probably required.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Typically, the reaction you are trying to carry out requires the presence of a strong acid. The role of the acid is two-fold: 1.) Hydrolyze the acetal
to the aldehyde and 2.) Catalyze the enolization of the rubeanic acid which is less reactive at sulfur than thiourea. My suggestion is to try the
reaction in aqueous methanol with an innocuous acid such as phosphoric acid. The acid concentration may have to be fairly high - not catalytic.
AvB
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Thanks for the replies guys! 
My intuitive response was to try a base because I have found that dithiooxamide reacts readily with hydroxylamine and hydrazine solutions to give
diaminoglyoxime and oxalimidazine (C2H8N6) respectively, however, in both cases hydrogen sulphide is eliminated.
clearly_not_atara, could I replace DBU with DABCO as I have the latter base?
Chloroacetaldehyde dimethyl acetal has a fairly low vapour pressure, is not a lachrymator and is stable so it scores over free chloroacetaldehyde.
I am going to try the acid catalysed process first because in the reaction between chloroacetaldehyde and either thiourea or thioacetamide hydrogen
chloride eliminated in the reaction combines with the resulting base, so I am going to try to add a little extra acid. I'll let you know how I get on.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Well I tried adding a little hydrochloric acid and another 20ml of methanol to the reaction mixture and refluxed for another 3 hours. The colour
remained the same orange red but copious amounts of orange red crystals formed. I am not sure whether these are the product or just dithiooxamide
crystallizing out of the reaction mixture. One promising sign though is they are fairly soluble in water and practically insoluble in methanol which
sound good for a dihydrochloride of 2,2-bisthiazole, the problem is they are bright orange and I would have thought they should be colourless. What do
people think?
 2,2'-bis(thiazole) dihydrochloride ?? 2,2'-bis(thiazole) dihydrochloride ??
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
"The comparison between thiourea and dithiooxamide thioamide and enol forms"
I would write "enthiol" or "thioenol" for A=C-SH sequence.
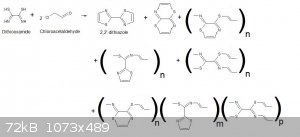
Beware that since dithiooxamide is bifunctional just like you chloroacetal that you may have different possible products...
1°) the product you want with two cyclopentarings...thus intramolecular cyclisation via geminal N and S on the
same C.
2°) a similar product with two cyclohexarings...thus intramolecular cyclisation via viccinal N and S from
different initially neightbourgs C.
3°) a polymer kind of linear with cycli inbetween (I can imagine two types) from the binding of different dithiooxamide molécules via intermolecular bridging.
4°) a mix of intramolecular cyclisation and intermolecular bridging with limited lenght and Molecular Weight.
The color is highly probable because of the hyperconjugaison of the molecule all sp2 are resonating and the free S doublets propagates the
"aromaticity".
[Edited on 12-8-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Isn't there some sort of reaction condition that naturally degrades thioamides? If you're trying to differentiate 2,2'-bisthiazole from dithiooxamide,
you can try doing something that would hydrolyze the latter and give oxamide; the arene should be significantly more stable.
Reading a few sources it seems like thiazoles form complexes with Cu2+ and this paper mentions something called "thiazole orange":
http://www.ncbi.nlm.nih.gov/pubmed/18066832
So you may be on the right track.
PHILOU's point, about the possibility of forming thiazino[2,3-b]thiazines, is also worth considering, though.
[Edited on 13-8-2016 by clearly_not_atara]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by PHILOU Zrealone  | "The comparison between thiourea and dithiooxamide thioamide and enol forms"
I would write "enthiol" or "thioenol" for A=C-SH sequence.
Beware that since dithiooxamide is bifunctional just like you chloroacetal that you may have different possible products...
1°) the product you want with two cyclopentarings...thus intramolecular cyclisation via geminal N and S on the
same C.
2°) a similar product with two cyclohexarings...thus intramolecular cyclisation via viccinal N and S from
different initially neightbourgs C.
3°) a polymer kind of linear with cycli inbetween (I can imagine two types) from the binding of different dithiooxamide molécules via intermolecular bridging.
4°) a mix of intramolecular cyclisation and intermolecular bridging with limited lenght and Molecular Weight.
The color is highly probable because of the hyperconjugaison of the molecule all sp2 are resonating and the free S doublets propagates the
"aromaticity".
[Edited on 12-8-2016 by PHILOU Zrealone] |
A little drawing is better than a long writting...and it can fix the ideas...so here is the general picture.
I have found 3 polymeric types and just thought to another mixed polymer possibility.
The polymer can be finite if the ends close a macrocyclic loop or "infinite" (if the chain propagates to very high MW).
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi c_n_a and Philou. I have done a good deal of research and reading since my previous post and it appears that the original method of producing
2,2-dithiazole was by heating 2-bromothiazole with copper powder at 150-170 C (Erlenmeyer & Schmid; Helv. Chim. Acta, v22 p698 (1939)). They
describe the dithiazole as a white solid melting at 102.5 C.
The strange orange product was obtained in 2.496g recovered and has several interesting properties. Evaporating down the filtrate produced a little
more impure material and finally a brownish oily residue that was rapidly decomposed and dissolved by sodium hydroxide solution. The first crop of
orange crystals is exceedingly well crystallized and apparently free of tarry or polymeric products. It is insoluble in cold water, sparingly soluble
in hot and a little more soluble in hot alcohols. It does not react with sodium carbonate suggesting that it is not a hydrochloride. I will check the
Mp tomorrow.
Do you think this sounds like c_n_a's thiazino[2,3-b]thiazine? Its certainly not 2,2'-dithiazole. It is interesting that in spite of the theoretical
possibility of forming polymers they are actually a very minor product at most.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Thiazino[2,3-b]thiazine is an interesting molecule: 1,4-thiazine fails to be aromatic because the 6-carbon is tetrahedral, but this double ring is
fully planar, so it should be an aromatic system.
Apparently nitrogen-sulfur bidentate ligands form Pd complexes which are useful in e.g. nucleophilic substitution at allylic esters:
http://www.sciencedirect.com/science/article/pii/S0022328X98...
https://www.researchgate.net/profile/Jacek_Skarzewski/public...
They also form complexes with Zn. So this compound may be quite an interesting ligand (even if it is not the one you expected)... this particular N-S
ligand is less bulky and possibly more stable than many others, and might (if you're really lucky) even be usable in hydrogenation, though
most other sulfur ligands will be reduced by those conditions so don't hold your breath.
| Quote: | | It is interesting that in spite of the theoretical possibility of forming polymers they are actually a very minor product at most.
|
Polymer formation is a decreasing-entropy reaction, so at "high" temperatures (which may be anywhere from 10-1000 K) it should proceed more quickly
backwards than forwards.
[Edited on 15-8-2016 by clearly_not_atara]
[Edited on 15-8-2016 by clearly_not_atara]
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
The old method is vapor volume versus molecular mass. If you can vaporize the stuff...?
That doesn't help with the hexagonal isomer; which was my first thought reading your post of a likely impurity. And - come to think of it, it looks
like something that would be colored as well...
So if you have failed to make the desired isomer you do have the chance to diazotize the amino thiazole and follow that with CuI / Cu(metal) - that's,
uh, Sandmeyer and Ullman coupling successively - should give you a small amount if all else fails.
One thing you might also try is to react the anions of thiooxamide with your acetal, then hit it with acid.
bis thiazole should form colored complex with metal copper or nickel. Or cobalt. Those complexes with the obvious isomer could be polymeric, so maybe
less soluble in things, because it's mainly the nitrogen the metal wants and there's a better geometry too. Could be a way of separating.
ARE you making it for purpose of a ligand? 
[Edited on 16-8-2016 by halogen]
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Thanks Halogen, I think your're right about the 2-aminothiazole route. I have prepared 2-aminothiazole and the route worked well but I think the
acetal route I used is faster and the reflux period in Vogel for the chloroether route is too long and you get a bit of brown tar that is difficult to
remove.
I have done some more investigation on my orange crystals. They form coloured complexes with Co, Ni and copper that exactly match those of
dithiooxamide... suspicious. So then I tried a melting point test, the powder suddenly turns black at 198 C and then begins to decompose with copious
evolution of hydrogen sulphide from 200 upwards to about 206 when decomposition apears to be complete. This fits the description of dithiooxamide
perfectly.
So it appears that I have recovered most of my dithioxamide and that very little reaction has taken place apart from possibly polymerisation of the
chloroacetaldhyde. I have found several more reaction of dithiooxamide in the literature and there is a strong common theme:- basic conditions. So I
am going to try this experiment again using a) the addition of a stochiometric amount of aqueous sodium hydroxide and b) a tertiary base, I am going
to try DABCO.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@halogen, re "One thing you might also try is to react the anions of thiooxamide with your acetal, then hit it with acid"
I just tried this with the carefully measure out quantity of NaOH in standard 1M solution on 1g of dithiooxamide to give the disodium salt and then
added the acetal. The dithiooxamide dissolved fairly quickly as I have observed before so I added a good deal of methanol to try and get the acetal
into solution. I then tried refluxing gently for 30 minute.
I got a very dark homogenous solution, curiously no salt precipitate, so I rendered it moderately acid and refluxed again for a brief period and
cooled. Filtering removed a very small amount of black water soluble grains. I evaporated off the methanol and got an oil. I tried triturating this
with a little ether and this caused the precipitation of a small amount of minute pale hairy ponpoms (filtered off about 40 mg). I then tried partial
neutralisation with sodium acetate and I am now waiting to see if anything is going to crystallise from the dark brown solution. If not I am going to
try some sodium hydroxide to free base any thiazole present. But it doesn't look promising . .
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The trouble is that 2-chloroethylidene dimethoxide is not nearly as strong an electrophile as 2-chloroacetaldehyde, which is an
alpha-haloketone, nor is it as reactive as 1,2-dichloro-3-oxapentane, which is an alpha-haloether. Both of these increase the reaction rate of
nucleophile exchange by a factor around 10^2-10^5 relative to a simple alkyl halide -- hence chloroacetone is more reactive than propyl chloride and
methoxymethyl chloride is extremely reactive as well.
However, in order to use the acetal as an electrophile before the acetal is destroyed, you have got yourself a quandary.
In this case you can cut the knot by preparing the alpha,beta-dihaloether. Methyl vinyl ether is the obvious choice, but there is no reason not to
use, e.g., benzyl vinyl ether, or isobutyl vinyl ether, but it is the benzyl compound I'll preoccupy the discussion with. Anyway, acetaldehyde reacts
with two equivalents of alcohol in the presence of concentrated H2SO4 to form a dibenzyl acetal:
MeCOH + 2 BnOH [H2S2O4] >> MeC(OBn)2H + H2O
This acetal is separated from the reaction mixture and destructively distilled with catalytic (clean) H2SO4 so that it fragments into benzyl vinyl
ether and benzyl alcohol:
MeC(OBn)2H (l) [cat H2SO4] >> H2C=C(OBn)H (g) + BnOH (g)
Fractional distillation separates the alcohol from the vinyl ether.
This then reacts with a halogen at low temperature:
H2C=C(OBn)H + Cl2 (CH2Cl2?) [-78 C?] >> H2ClCC(OBn)ClH
to give the alpha,beta-dichloroether. I am familiar with the preparation of vinyl ethers, and I of course expect chlorine to react
preferentially with alkenes at least at low enough temperatures, but I haven't actually seen a reference for the last part of this tek, so you may
want to look around for one. However, I believe that something like this will achieve the preparation of your haloether, and, therefore, your
heterocycle.
[Edited on 17-8-2016 by clearly_not_atara]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I just thought the fact that thiazole and/or thiazine moeties could be involved into Diels-Alder type of cyclisations (cage tricyclo
compounds)...Right? So problem might be more complex.
I have rewritten the equations of clearly_not_atara for convenience purpose...I hope he/she doesn't mind. 
CH3-CH=O + 2 C6H5-CH2OH --H2S2O4--> CH3-CH(-OCH2-C6H5)2 + H2O
CH3-CH(-OCH2-C6H5)2 --cat H2SO4--> CH2=CH-OCH2-C6H5 (g) + HOCH2-C6H5 (g)
CH2=CH-OCH2-C6H5 + Cl2 --CH2Cl2/-78 °C--> Cl-CH2-CHCl-OCH2-C6H5
[Edited on 17-8-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|