Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
ethylbenzene by Wolff-Kishner (Huang-Minlon)
I intend to do this preparation shortly but am perplexed by the procedure in Vogel's 3rd ed, p. 516. Here is the procedure:
"Place 36.0g of redistilled acetophenone, bp 201°, 300mL of diethylene glycol, 30mL of 90% hydrazine hydrate and 40g of potassium hydroxide pellets
in a 500mL Claisen flask provided with a reflux condenser and a thermometer dipping into the liquid. Warm the mixture on a boiling water bath until
most of the potassium hydroxide has dissolved then reflux (free flame) for one hour. Arrange the apparatus for distillation and distill until the
temperature of the mixture rises to 175° (note 1); keep the distillate (ca 50 mL). Replace the condenser in the flask and continue the refluxing for
3 hours.
Separate the upper hydrocarbon layer from the distillate and extract the aqueous layer twice with 20mL portions of ether; dry the combined upper layer
and ethereal extracts with anhydrous magnesium sulfate, remove the ether on a water bath and distill the residue from a 50mL Claisen flask. Collect
the ethylbenzene at 135-136°; the yield is 20g. By extracting the syrupy liquid in the reaction flask with three 30mL portions of ether a further 2g
of ethylbenzene, bp 136°, may be obtained.
Note
1. The reduction takes place at low temperature and is fairly rapid for acetophenone. With higher ketones the upper layer of the initial distillate
should be returned to the contents of the reaction flask and refluxing continued for 3-5hrs. The reactant mixture and aqueous distillate are then
combined, extracted with ether, etc. "
It is fine up to the point where the pot residue is refluxed for another 3 hours. But then nothing is done with this product. Only as almost an
afterthought is it mentioned that another 2g of ethylbenzene can be extracted from the "syrupy residue."
Am I missing something?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
I do not think that the additional reflux is only done to give an extra 10% in yield. It appears that the additional reflux time is necessary for
higher homologs of acetophenone. This comes from parsing Note 1.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I agree.
Quote: Originally posted by AvBaeyer  |
It appears that the additional reflux time is necessary for higher homologs of acetophenone. This comes from parsing Note 1.
|
I agree with your reasoning here. But doesn't it look like Vogel was a little sloppy in his presentation of this procedure for ethylbenzene?
I have looked up the original work by Huang-Minlon in JACS. There are two articles, the first in 1946, volume 68, shows preparations for
n-probylbenzene and cyclohexane. These also use the 3hr addtional reflux. In these cases the distillate and the residue are combined and extracted
with ether.
The 2nd article shows how this procedure can be used to reduce nitro groups, 1948, volume 70.
Attachment: Wolf-Kishner (Huang-Minlon) 1946.pdf (281kB)
This file has been downloaded 464 times
Attachment: Wolff-Kishner (Huang-Minlon) nitro reduction 1948.pdf (498kB)
This file has been downloaded 595 times
[Edited on 25-8-2016 by Magpie]
[Edited on 25-8-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
I find a good deal of Vogel to be sloppy and sometimes less than fully correct. Best to find reaction conditions in Org Syn, which Vogel largely
plagiarizes anyway. That's not to say that Vogel does not have utility, which in general it does. Never in my professional career did I see anyone
using Vogel as a primary reference. Just my rant.
Now to be more helpful. There is a synthesis procedure for 4-methylethylbenzene in Organic Reactions, vol 4, p 389 which is in the forum library. Not
exactly the procedure you found in Vogel but looks good. See also JACS, 68, 2487 (1946) which is attached.
AvB
Attachment: Wolff-Kishner Redn huang-minlon1946.pdf (281kB)
This file has been downloaded 490 times
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
I did not see that you had already posted the article that I attached. Sorry.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
So I think what you are saying is that for ethylbenzene I don't need to do the 3hr reflux unless I want the 2g in the syrup? Or maybe its in the
syrup without the 3hr reflux?
What worried me about extracting the syrup was its large volume and the fact that DEG and DCM (my substitute for ether) are mutually soluble. So I
did a test tube experiment: I added 1mL of DCM to 1mL of DEG. They are indeed mutually soluble. Then I added 1mL of water. Again everything was
mutually soluble. Then I added ~30mg of KOH, which went into solution. Then 2 phases appeared: a larger upper phase and about 1mL of the lower
phase. So I conclude that extracting the syrup would be practical. Do you agree?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
I'd extract it, perhaps keeping it separate from the distillate, and see what you get - no harm in trying to gain that extra bit of material. At the
least, hold on to the syrup until you're sure you've isolated most of the product in the distillate, so that you can process the syrup further if you
find that you're missing the material you expected.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
*So I think what you are saying is that for ethylbenzene I don't need to do the 3hr reflux unless I want the 2g in the syrup? Or maybe its in the
syrup without the 3hr reflux?*
The extra 3 hrs of reflux are probably not productive for ethylbenzene. I would not fight for the extra 2 grams of product, with or without extra
reflux, if the initial distillate gives you an acceptable yield. It's going to be a messy extraction. In my opinion, it comes down to time value.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
AvB you are right, of course. But I decided to just follow the procedure as I had the time, and the ice. The 3hr reflux was finished this afternoon.
The distillate from the initial distillation looks as expected both in quantity and in showing 2 phases nicely. Tomorrow I'll do the extractions. If
the extraction of the syrup (over 300mL) goes OK I'll combine it with the other extraction and the hydrocarbon phase of the distillate, dry, and then
distill again to get the ethylbenzene. If the extraction of the syrup looks like junk I will simply discard it.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
Curious to learn how this all worked out for you.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
AvB,
There will be a full report in a day or two. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Ethylbenzene by Wolff-Kishner reduction of acetophenone
Ethylbenzene was produced via a Wolff-Kishner (Huang-Minlon) reduction of acetophenone following the procedure in Vogel (ref 1).
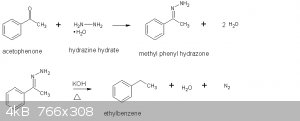
The Wolff-Kishner reduction exists in an alkaline medium. This provides a valuable alternative to the Clemmensen reduction which exists in an acid
medium.
1. Reagents
36g of freshly distilled acetophenone
34mL of 79.6% hydrazine hydrate
300mL of diethylene glycol (DEG)
40g of KOH
dichloromethane (DCM) in place of ether
anhydrous magnesium sulfate
The DEG can be obtained OTC as a cooking fuel for camping. A picture of the two cans I used is shown below:

Couglan’s camp heat
2. Procedure
A 500mL RBF was setup with an electric heating mantle and a magnetic stirrer. A Claisen adapter was attached. In the center neck a thermocouple was
installed to allow temperature measurement of the liquid. To the long neck was attached a condenser.
The flask was loaded with the DEG, acetophenone, hydrazine hydrate, and KOH in that order. Heating was begun but stirring was not possible until the
KOH had sufficiently dissolved a short time later.
a. initial reflux Continued heating brought the yellow product mix to a steady reflux for 1 hour. Photos of the lagged and un-lagged
apparatus are shown below:

lagged reflux

un-lagged reflux
The un-lagged picture shows the yellow color of the intermediate hydrazone.
b. initial distillation After 1hr the condenser was reconfigured to allow downward distillation. This was continued until the pot liquid
reached 175°C. Something over 50mL of 2-phase distillate was collected as shown below:
Attachment: phpqz9s4H (139kB)
This file has been downloaded 844 times
initial distillation picture
c. final reflux The condenser was again configured for reflux. This was continued for 3hrs. Following this it can be seen that most all
of the hydrazone has been converted to ethylbenzene as the yellow color is virtually gone. After cooling to room temperature this had the consistency
of corn syrup.

Wolff-Kishner syrup.
The EPDM rubber stopper used as a thermocouple adapter was heavily degraded as can be seen in the picture below.

TC adapter
d. solvent extractions
1. The aqueous layer from the initial distillate was extracted with 2x20mL of DCM. The extract was combined with the hydrocarbon phase from the
initial distillate in an Erlenmeyer flask. A scoop of anhydrous MgSO4 was added for drying.
2. An extraction of the syrup was also attempted using 50mL of DCM. However, no phase separation occurred. Four test tube tests using 2mL ea of the
syrup were made under the following conditions:
1. 1mL of water was added
2. K2CO3 was added
3. 1mL of water and K2CO3 were added
4. 1mL of ether was added
Only test # 4 using ether produced a phase separation. Therefore I added 30mL of ether (all I had on hand) to the syrup and attempted an extraction.
The ether phase separated but by the time I returned to the hot lab most of the ether had evaporated. Not wishing to pursue this any further,
extraction of the syrup was abandoned. However, I feel that the 2g of ethylbenzene reported by Vogel is indeed likely to be in this syrup. My
conclusion is based on the color change of the syrup from yellow to colorless following the 3hr reflux.
e. 2nd distillation
The dried extract/distillate was again distilled. The fraction boiling in the range 135°-136°C was taken as ethylbenzene.

Distillation of ethylbenzene

Ethylbenzene boiling point

Ethylbenzene in receiver
2. Results
25.0g of ethyl benzene was obtained for a % yield of 78.5%. The Vogel expected yield is 20g.
3. Discussion
There was some confusion on my part concerning the procedure as presented by Vogel. This is discussed earlier in this thread. In the end I decided
to follow the procedure “as is” except I used DCM in place of ether. Although time consuming, this is a facile method for the reduction of
acetophenone. And according to Huang-Minlon it works well for the preparation of n-propyl benzene and cyclohexane (ref 2) as well.
4. References
1. Practical Organic Chemistry by Arthur I. Vogel, 3rd ed, (1956), p. 516
2. “A Simple Modification of the Wolff-Kishner Reduction,” by Huang-Minlon, JACS, 68, (1946), pp. 2487-2488.
[Edited on 27-8-2016 by Magpie]
[Edited on 27-8-2016 by Magpie]
[Edited on 28-8-2016 by Magpie]
[Edited on 28-8-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's a good reason not to reflux the reaction mix for 3hrs. It seems the hot (183°C) alkaline mix permanently etched my 500mL RBF. I have tried
alcoholic NaOH and chromic acid cleaners to no avail.

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
Your degraded stopper makes me think of the original Huang-Minlon variation. If I'm not mistaken he discovered this method when he did a Wolff-Kishner
reduction with a cork where you had the rubber stopper, and the cork degraded leaving a gap where the vapors were able to slowly escape during reflux.
He came back to find what he thought was a ruined reaction, but worked it up anyway and got a great yield. If memory serves me right I found that
anecdote in Feiser's TOPICS IN ORGANIC CHEMISTRY, an entertaining read, but maddening in a way because it's a supplement to another book which I don't
have, so some of the chapters start in the middle of the story.
I'm still hoping that somebody else with a hyphenated name discovers a useful variation on this process we can have a reaction with an even more
imposing name.
|
|
|