Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
aldol condensation....
Someone I know has been trying to perform a acidic crossed aldol between benzaldehyde and MEK... He prefers avoiding generating dry HCl, although he
has tried it a few times... He saw a post from twodogs mentioning he used H2SO4 and HCl at reflux although he "didn't obtain the same results as with
dry HCl"
During the first trials with dry HCl, everything happened as mentionned, color changes, smell, etc, temperature was mentained between 0 and 5°C and
gassing was done very slowly for at least 3 to 5 hours... 12h after, the dark black-reddish mix was washed with 10%NaOH, washes were white, then sat.
NaCl, washes were yellow, and plain dH2O, washes were very slightly yellow and then transparent... Organics were dried over Na2SO4, and distilled to
get rid of remaining MEK.... after temp. starting to go over 85°C, the black residu was cooled in ice bath, and nothing crystalized.... another trial
was done the same, but the organics were not distilled, and directly cooled. A certain amount of crystals apperaed after a few minutes, they were
suction-filtered (seemed to be less then!) and were slightly yellowish-white. After reading Bio's experiments, they were washed with 96% EtOH, and
this clearly disolved some crystals, the EtOH was in the buchner with the original organic... this was distilled, and residu cooled, no crystals...
the temp never excedded 85°C (head temp.).... about 1.5g MPB with 30g Benzaldehyde..... hum..
Next, a few experiments were tried at reflux... 1 to 2 molar ration of Bz to mEK, with conc. HCl et Conc. H2So4 seperatly, post
reaction as before, cooling and no crystals... With H2SO4 (slowly dripped in over a few hours), colour change was very quick, each drop of acid turned
blood-red instantly... reaction mixture was very dark red/black after 2 hours.. With HCl, colour change was more gentle, and after 2 hour RM was blood
red... To mention: benzaldehyde smell was noticed in organic after distillations... retroaldol? though they were washed and dried.... One reaction
cold-Dry HCl was left overnight in ice bath to heat up slowly, but condensation on the sides of beaker gave a aqueuse phase next morning....
benzaldehyde smell and no crystals...
One experiment was done at -5 to 0°C with H2SO4 dropped in on the course of the reaction.. no results..
Reaction was tried with a Dean-Stark trap, 1 to 2 molar Bz/MEK, and about 75mL toluene, with conc.HCl expected about 6.5mL H2O, counting water from
the acid, and obtained. organic, who was golden yellow/orange, was washed as previously and treated with Bisulphite (he didn't want to distill all
that toluene, seeing was distillation did previously...), alot of white adduct formed, was washed with alcohol and ether, then nutralised with
10%NaOH, extracted with 2*50mL DCM, washed with 10% HCl and 3x H2O(organic was light yellow), and DCM distilled. Colour became dark orange. Organic
cooled down, no crystals... worth to mention: when washing the original organic, solvant on dean stark evaporated, and gave some white crystals, the
crystal pattern ressembling the MPB alot... not enough to analyse... didn't check if they melted under hot lamp, very stupid... an experiment with
DeanStark and 1mL conc. H2SO4 was started, after 1H30 of reflux, 2.5mL H2O obtained, on 6 expected, but had to stop. The reaction mixture went from
light yellow to orange to very dark black/red as in the HCl experiemnts... Reaction not completed for the moment...
Any advice or comments would be greatly welcome, from anyone with experience with this reaction or crossed aldol in general.... Distillation seems
to destroy the product... will try a 1 to 1 molar ratio and cool after washes to see..looks so simple, but... a lot of effort, time and reagents was
put into this...
P.S. all reagents are AR grade..... and WTF happened to synthetikal? I was gone for a few months...
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
bump.. would really appreciate some help... thank you
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
What was the question?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Sorry, it's true I didn't express it very well 
What could be the causes of these failures?
Can anyone who has already tried this reaction or a similar one explain their procedure in detail?
Has anyone a protocol on simple acidic crossed aldol condensations with similar reactants, using a dean stark or not? (I have already a few extracts
cited on related posts of the Hive, Synthetikal, but not enough details to understand what's going wrong...)
Can anyone HELP?
What happened to Synthetikal.com?
Thank you
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
The procedure you want to follow is on Lambdasyn. They use aceton instead of MEK. Follow that one, it should work.
If you're interested in the reaction details, look here:
1. aldol addition: http://www.organic-chemistry.org/namedreactions/aldol-additi...
2. aldol condensation: http://www.organic-chemistry.org/namedreactions/aldol-conden...
damnant quod non intelligunt
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thanks for thoses links, but I've already seen them, to understand all the mecanics of enol formation etc.. I was looking for something with detail
towards the protocol itself, I gonna search the lambdasyn portal, but isn't that in deutch? I'm not much of a german speaking.... thanks for the help
anyway
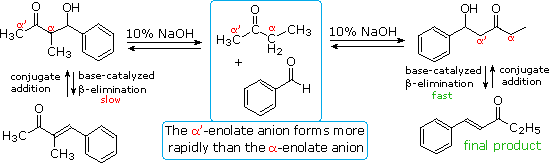
EDIT: I check the procedure on Lambdasyn, thta's for benzylidenacetone, and it's a BASIC crossed aldol, that would unfortunaly lead to another
product with MEK, base catalysed aldol goes by an enolate ION, and with MEK, the most stable enolate ion would form on the carbon of the methyl chain,
and not the (alpha) carbon of the ethyl chain... this would give C6H5-CH=CH-C(=O)-C2H5 as main product and not
C6H5-CH=C(-CH3)-C(=O)-CH3
By the way, another reaction was tried, with a 1 to 1 molar ration, dry HCl, and unfortunaly, one had to leave the lab for a certain moment because of
unexpected problem, and when he came back, to his horror, the HCl generator had sucked back half of the reaction mixture who was already dark
red......... so half of the mixture was in the H2SO4 drier, as a dark layer, and when he unplugged the tube, immediately started bubbling and turning
in horrible black tar (as soon as oxygen enter, I suppose) that was a real pain in the ass to get rid of....... the next morning, the other half, who
was intact and had been stirred all night, was washed, and then put in freezer, only cristals of water apperead (had not being dried), but after
addition of an equal volume of ether, some cristal appeared.... very very low yield, however, even considering half of the mixture had gone....
slowly slowly getting there perhaps? I'd never think the reaction would cause some much difficulties.... evryone else seems to find it outrageously
easy!! and he didn't start practicing chemistry 2 weeks ago...
[Edited on 23-10-2006 by Klute]
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | EDIT: I check the procedure on Lambdasyn, thta's for benzylidenacetone, and it's a BASIC crossed aldol, that would unfortunaly lead to another product
with MEK, base catalysed aldol goes by an enolate ION, and with MEK, the most stable enolate ion would form on the carbon of the methyl chain, and not
the (alpha) carbon of the ethyl chain... this would give C6H5-CH=CH-C(=O)-C2H5 as main product and not
C6H5-CH=C(-CH3)-C(=O)-CH3 |
As far as my knowledge of aldol chemistry reaches (it has been a long while since I've performed practical experiments with it), I think you're wrong
here. When you have an asymmetrically disubstituted carbonyl (like MEK), you have to make a clear distinction between the thermodynamically and
kinetically most stale enol.
In the case of MEK, the kinetic enol is formed with the methyl side chain; the thermodynamic enol is formed with the ethyl side chain. Unless you take
the necessary precautions (capturing the kinetic enol as its trimethylsilyl ether, working at cryogenic temperatures, ...), you'll be working with the
thermodynamically most stable enol. This is the enol with most substituents at the alkene bond (allows hyperconjugation, hence stabilization). So as
far as I understand it, I suspect that most of the MEK-enol will add to the benzaldehyde's carbonylic carbon with the enolizable carbon from the ethyl
side chain (when working at room temperature and above).
damnant quod non intelligunt
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Actually, with the base catalyzed condensation one always gets the kinetic product, since the attacking nucleophile is the enolate formed by the
deprotonation of the most acidic hydrogen. (The hydrogens on the CH3-CO- side are considerably more acidic than those on –CO-CH2-Me side. So the
product with the usual base catalyzed condensation would be Ar-CH=CH-CO-Et.)
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | Originally posted by Nicodem
Actually, with the base catalyzed condensation one always gets the kinetic product, since the attacking nucleophile is the enolate formed by the
deprotonation of the most acidic hydrogen. (The hydrogens on the CH3-CO- side are considerably more acidic than those on –CO-CH2-Me side. So the
product with the usual base catalyzed condensation would be Ar-CH=CH-CO-Et.) |
And after equilibrium? The thermodynamic product is more stable then the kinetic one, so unless you work at cryogenic temperatures and/or capture the
kinetically formed enolate as a trimethylsilyl ether, I thought you'd end up with the thermodynamically formed enolate, which eventually will be the
reaction partner.
damnant quod non intelligunt
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
yes, nicodem is right about the enolate ION, acidic aldol proceds by a straight enol.... this reaction is an equilibrium, I do get your point of view
Sergei, but from all that I've read, this is the way it happens... I beleive the speed of deshydratation also interferes with the major final
product...
|
|
|
twodogs
Harmless

Posts: 10
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Klute
If I was you I would forget about the H2SO4 and conc HCl and just use the dry HCL. It is a good idea to weigh the combined reactants before the
gassing and then you can determine how much HCL has been added. Too little or too much affects how much of the unsaturated ketone you get. Forget
about all that washing as well...it is not necessary.
Once the required amount of HCL has been added, leave overnight and then distill off the excess MEK, then distill and collect the unsaturated ketone
at atmospheric pressure if you want. You will collect a yellow/golden oil. Put this in the freezer for a few hours and if no crystals form try this.
Put a metal spoon in the freezer for an hour or so. Quickly dip the cold spoon in and out of the very cold oil . A few crystals will form on the
surface of the spoon. Drop those crystals back into the oil and that should induce crystallization.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I would like to ravive this old thread just to add that twodogs was right: nothing beats gaseous HCL. I have done numerous trials with various acids
(H2SO4, conc HCl, TsOH, H3BO3, etc) with dean stark and without, and nothing even compares to gaseous HCL. Routine yields are 70%, ei 1g MPB for 1g
benzaldehyde introduced.
The unsaturated ketone has been hydrogenated using CTH 10% Pd/C and ammonium formate, and gives a very nice smelling product, there is a patent
(US5372995) on the condensation with conc H2SO4 (no yield stated IIRC, unreacted benzaldehyde present) and on the hydrogenation over Raney Nickel.
I will take some pictures next time I do this (if ever).
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
|