NzForever
Harmless

Posts: 1
Registered: 6-10-2016
Member Is Offline
Mood: No Mood
|
|
Chemical Bonds
So I live over in NZ and we have a stupid system for school called NCEA. Anyone I've been watching certain youtube videos, one is from this guy over
in america and his stuff is quite detailed and its reliable and knowledgeable.
The other youtube channel I've been watching is a local youtuber, who does videos specifically for our NCEA exams. Anyone if there are any chemistry
experts out there, it'd be great if you could help clear a few things up for me, just some stuff in this guys video doesn't seem right. For reference
the video is :https://www.youtube.com/watch?v=VW6B1zjxoM0&t=22s
Anyway, some things he said were that convalent bonds are always non-polar and that the 4 types of bonding we have to learn are
convalent, ionic, metallic & intermolecular. This seems to be the way it goes for our exams also, because I've been doing the
practice tests.
The thing I don't understand is that ain't the main types of bonds Convalent, Ionic and Metalliac the main sort of bonds? and intermolecular is a
force not bond? It would help if someone explained bonding and forces as simply as you can.
Ive attached a couple of images from our practice tests, please clarify if these answers are right?
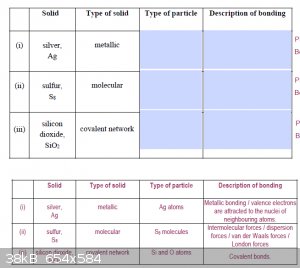 
[Edited on 10-7-2016 by NzForever]
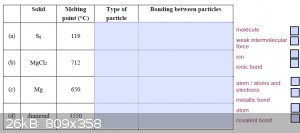
[Edited on 10-7-2016 by NzForever]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
That's wrong. Covalent bonds can be either polar, like H-Cl, or non-polar, like Cl-Cl. In the polar HCl molecule, the more electronegative chlorine
atom pulls electron density towards it more strongly than the hydrogen atom does, creating a permanent dipole. In other words, the uneven sharing of
electrons between the two atoms results in a partial-positive charge on the hydrogen and a partial-negative charge on the chlorine. In the diatomic
Cl2 molecule, the two chlorine atoms are pulling electron density away from one another more or less evenly, resulting in a non-polar
covalent bond.
| Quote: | | The thing I don't understand is that ain't the main types of bonds Convalent, Ionic and Metalliac the main sort of bonds? and intermolecular is a
force not bond? |
Technically, they're all forces, and they all rely on electrostatic attraction and repulsion. The difference is that covalent, ionic and metallic
bonds are intramolecular forces (or "bonds"), while dipole-dipole interactions (like the two water molecules in the Youtube video), London dispersion
forces and hydrogen bonds are intermolecular forces (or "bonds").
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
A lot of what that video says (in the 50% I actually watched) is a not-bad simplistic and clear explanation. But there is enough crap in there that
is just straight out wrong. You should not trust it.
NCEA started as a fetid pile of crap. It seems a decade and a half on that it still is. And if this is an example of the resourcing that is thrown
at it then it does not inspire much confidence.
For good clear explanations that are detailed and accurate I recommend http://www.chemguide.co.uk/
As a teacher I would never talk about intermolecular bonding in the same discussion as metallic ionic and covalent bonding. I think it invites
confusion and conflating of the principles involved. And he omits the fact that there are several kinds of intermolecular attractions. It is
important to realise that not all intermolecular forces are the same strength. This has a huge impact on physical properties. If you are going to
have any grasp of the relationship between bonding and properties then this is essential.
What else specific is wrong in the video?
He says that ionic bonding is usually between a metal and a non-metal. I would unhesitatingly drop the word "usually". The only common
counterexamples are ammonium salts.
But it is imprecise phrasing. Ionic bonding is between cations and anions rather than between metals and non-metals. Understanding ionic substances
is contingent on understanding the formation of ions. Despite superficial similarities, ions are very different from atoms: they are different sizes
and behave very differently. It is the dissimilar charges on the ions that causes the bonding that holds the ionic lattice together. The attractive
force is not between atoms but between ions. For a good grasp of ionic substances watch this clip. I instruct my students to watch it half a dozen times or more until they can nearly quote it. The wording is very clear and every
word important and carefully chosen.
He states that MgO is an ion. That is flat-out wrong. I have no idea what he is on about there.
There were a couple of other glitches but I am not re-watching to find them.
[edit]
fixed link
[Edited on 7-10-2016 by j_sum1]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I would forget about the whole phrase "usually between metals and non-metals". I would phrase it as follows:
- Ionic bonds are between cations (positively charged) and anions (negatively charged).
- Frequently encountered cations are positively charged metal ions, but other types of cations do exist (e.g. ammonium ion).
- Frequently encountered anions are negatively charged non-metal ions, but other types of anions do exist. Many anions are composite entities, having
multiple atoms, covalently bonded, with the entire entity having a negative charge (e.g. sulfate ion, nitrate ion).
I would say that there are following bonds:
- covalent: sharing of electrons by different atoms. Example: HCl, one electron donated by H atom and one electron donated by Cl-atom, the bond
incorporates two electrons.
- ionic bond: two (or more) different charged species are combined in a single lattice and electrostatically remain together. Example:
Na<sup>+</sup>Cl<sup>-</sup>, usually simply written as NaCl. The charged entities themselves can be composite, with covalent
bonds in the entities, the complete entities being charged, example: (NH<sub>4</sub><sup>+</sup> <sub>2</sub>SO<sub>4</sub></sub><sup>2-</sup>, usually simply written as (NH<sub>4</sub> <sub>2</sub>SO<sub>4</sub></sub><sup>2-</sup>, usually simply written as (NH<sub>4</sub> <sub>2</sub>SO<sub>4</sub></sub> <sub>2</sub>SO<sub>4</sub></sub>
- metallic bond: a positively charged species (almost always a metal ion, but this need not be so), kept together by a counterbalance of freely moving
electrons in a so-called 'electron sea'. The positive ions and the negatively charged electrons keep the whole thing together. We perceive such
'electron sea' as a shiny surface, a typical metallic appearance.
- dative bond: This in fact is not much different from a covalent bond, but with this dative bond, the shared electrons are all donated by one of the
partners, involved in the bond. An example is the ammonium ion, in which H<sub>3</sub>N: shares its two free electrons on the nitrogen
with a H<sup>+</sup> ion in order to make H<sub>3</sub>N:H<sup>+</sup>, the common ammonium ion
NH<sub>4</sub><sup>+</sup>. Other common examples are hydrated metal ions, e.g. Cr(:OH<sub>2</sub> <sub>6</sub><sup>3+</sup>, in which two electrons of the water
molecules on the oxygen atom are shared with the Cr<sup>3+</sup> ion. Dative bonds can also exist between neutral species, e.g. in
F<sub>3</sub>B and :NH<sub>3</sub> forming the tightly bonded molecule F<sub>3</sub>B:NH<sub>3</sub>.
Here :NH<sub>3</sub> is the donor of both electrons. <sub>6</sub><sup>3+</sup>, in which two electrons of the water
molecules on the oxygen atom are shared with the Cr<sup>3+</sup> ion. Dative bonds can also exist between neutral species, e.g. in
F<sub>3</sub>B and :NH<sub>3</sub> forming the tightly bonded molecule F<sub>3</sub>B:NH<sub>3</sub>.
Here :NH<sub>3</sub> is the donor of both electrons.
More types of bonds exist, but these are more exotic. An example is the so-called 3c-2e (three center, two electrons) bond in diborane, in which two
electrons are donated by two atoms, while these electrons are shared by three atoms. Even more exotic bonds exist in ions like
Te<sub>4</sub><sup>2+</sup>, but these kinds of bonds are not common and beyond the scope of standard introductory courses.
These exotic bonds in fact also are covalent, but the bond is not between two atoms, sharing a pair of electrons, but between more than two atoms,
sharing two or more electrons.
Finally, things like inter-molecule forces I consider as different from the above types of bonds. These forces do have a large influence on the
physical properties of materials though.
The bond types, mentoned above, all are kind of 'extremes'. In practice, things may be not as simple as what is written above. A covalent bond may
lead to asymmetric charge distribution over the molecule. As already pointed out, in e.g. HCl, the negative charge is concentrated somewhat more
strongly around the Cl-atom, so the two shared electrons are not equally shared, the Cl-atom has them more 'around it' than the H-atom. In some
covalent compounds the charge distribution may be so asymmetric that one hardly can speak of a covalent bond anymore and the bond is almost ionic. An
example of this is a compound like anhydrous CuCl2 (a brown/yellow solid). Many people consider this a salt (having ionic bonds between copper(II)
ions and chloride ions), but in fact it still is covalent, but with the negative charge almost completely drawn to the chlorine atoms. In many
solvents, this compound breaks down in ions and you get a conducting solution. Such solutions tend to be blue, the common color of solvated copper(II)
cations. In some solvents (e.g. acetone) this compound dissolves as molecules CuCl2 and such solutions are brown/yellow. The lesson to be learned from
this example is that there is a gray area between bond types. Another borderline compound between ionic and covalent, but somewhat less pronounced, is
HgCl2. Solutions of this in water still consist of HgCl2 molecules, but there also is quite some Hg<sup>2+</sup> and
Cl<sup>-</sup> in solution.
There is also a gray area between bonds and intermolecular forces. An example is solvation of sodium ions in water. Water molecules have covalent
bonds, but the charge is not evenly distributed. The oxygen atom has a somewhat negative charge and the hydrogens have a somewhat positive charge. The
positively charged sodium ions attract water molecules on their oxygen-side. The oxygens have two free electrons and share these with the sodium ions.
This bond, however, is quite labile and is easily broken. So, a certain water molecule is not really solid fixed to a certain sodium ion, but still,
one can speak of a dative bond to some extent. Here we have an example of a gray area between dative bonds and intermolecular forces. If we replace
the sodium ions with trivalent chromium ions, then the picture changes quite a lot. The chromium ions attract the oxygen side of the water molecules
much more strongly than the sodium ions do and once a water molecule has shared its two free electrons on its oxygen atom with the chromium metal ion,
then it is tightly bound and cannot escape anymore. The electron withdrawing power of the chromium ion is so strong that the negative charge is going
deeply inside the complex ion and the hydrogens on the water molecule become almost completely positively charged. Some of these hydrogen ions
actually split off as H<sup>+</sup> and the solution becomes acidic.
[Edited on 7-10-16 by woelen]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The Main thing to remember is that you're learning the Correct answers to the Questions that you will be tested on so that you pass
the exam.
This may not be technically 100% correct in the real world.
If you give the currently-understood 100% Right answer, likely you'll get a Fail for that question because it was not the the answer they wanted.
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Yeah but nah aga.
The examination snd the revision resource are from two different providers. (And the exam markers are different again - contracted school teachers.)
Like I said, NCEA is a mess.
[edit]
Nice post woelen. I have not come across the tetratellurium ion before. That sounds like a bundle of fun.
[Edited on 7-10-2016 by j_sum1]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The tetratellurium ion can be made easily. Add some solid tellurium to concentrated H2SO4 and gently heat, so that the acid becomes warm (need not be
really hot). The tellurium dissolves with a beautiful intense pink/red color, which is the color of the Te4(2+) ion.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by j_sum1  | | The examination and the revision resource are from two different providers. (And the exam markers are different again |
That sounds totally insane.
I guess it requires a whole army of publicly funded admin staff, which would explain the 'sense' behind it.
-----------------------------------------------------------------------
Happily, here at B&D Uni there are no admin staff, excellent Tutors and courses, plus well administered exams 
B&D now operate as a fully Open and Transparent outfit.
(i stole all the firedoors, a few hundred roof tiles and eight wall panels last week. U2U while stocks last !)
|
|
|
|