Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
4,7-phenanthroline-5,6-dione intermediate
I am trying to prepare some heterocyclic ligands containing fused pyridine elements but a key intermediate is the above name compound. I have found a
paper (1) that describes the preparation in detail from the compound 2-methoxy-1,4-phenylenediamine which was once widely available as a component of
hair dye, however, possibly due to concerns over possible carcinogenic properties it has dissapeared from the market and I can't find a preparation
for this compound. I have even searched the patent literature and to my surprise (given its past use) I could fine nothing.
So how do you prepare it?
I was wondering if it can be prepared by nitrating 2-anisidine (2-amino-methoxybenzene) to give 2-amino-4-nitro-methoxybenzene which can then be
reduced to the required diamine? The question is therefore if I were to nitrate 2-anisidine where would the nitro group enter? Or put it another way
which of the two starting functional groups is the more strongly o-p directing? My "gut" feeling is that the amino group is if protonated by a
sulphuric acid medium (consider nitration of aniline).
I can see various other possibilities such as nitration of 3-methoxy-aniline (m-anisidine), here both function group should activate the same
positions and so hopefully the main product would be 3-methoxy-4-nitroaniline but in practice there are three possible mononitration products here. A
similar route with similar orientation issues exist for 3-aminophenol.
Orientation issue could be avoided by starting with 2-hydroxy-terephthalic acid dimethyl ester via O-methylation, amidation and a double Hoffman
degradation.
If anyone with access to reaxys or scifind could help it would be very much appreciated.
(1) Imor et al.; Synthetic Communications; 1996; v26; issue11; pp 2197-2203
[Edited on 11-12-2016 by Boffis]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
start off with 3-nitroanisole(you could start with 3-nitrophenol,but better to avoid the methylation  ) and nitrate it to 2-methoxy-1,4-dinitrobenzene,then reduce it using dithionite to the target compound. ) and nitrate it to 2-methoxy-1,4-dinitrobenzene,then reduce it using dithionite to the target compound.
nitration of 3-nitroanisole to the dinitro-http://onlinelibrary.wiley.com/doi/10.1002/recl.19030220803/... (pg 270)
methylation of 3-nitrophenol using methyl iodide-http://www.sciencedirect.com/science/article/pii/S0960894X09...
I have a little doubt in the reduction step.Chemplayer made a video showing the reduction using dithionite where the mixture is heated for sometime to
completely reduce the substrate.I have also seen other procedures which claim that heating is required. But in Fieser's synthesis of martius
yellow,just stirring the 2,4-dinitro-1-naphthol with dithionite at room temperature is enough for complete reduction.Also fieser's reduction takes
5-10 minutes compared to chemplayer's 30 minutes even though the former compound has 2 nitro groups whereas as the latter has 1.I attributed this
anomaly to the OH group of 2,4-dinitro-1-naphthol which activates the ring,but I may be wrong.Can someone explain ?
chemplayer's video-https://youtu.be/1PXmg2OZDmI?t=656
fieser's reduction method-http://www2.chemistry.msu.edu/courses/cem415/CEM415,%202014/... (pg 5)
Chemplayer makes the dithionite in the video but you can get it directly too
Quote: Originally posted by Magpie  |
Na2S2O4, sodium dithionite (aka sodium hydrosulfite), is available from DanielSmith Co. It should also be available locally as a Rit dye (Rit Color
Remover). Try your grocery store. |
[Edited on 11-12-2016 by CuReUS]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Many Thanks CuReUS!
That's wonderful, I even have the materials (3-nitrophenol and methyl iodide at least). This is going to be some fairly challanging chemistry!
Boffis
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Boffis  | | The question is therefore if I were to nitrate 2-anisidine where would the nitro group enter? Or put it another way which of the two starting
functional groups is the more strongly o-p directing? My "gut" feeling is that the amino group is if protonated by a sulphuric acid medium (consider
nitration of aniline). |
Protonation of the amino group will cause it to become deactivating and thus meta-directing. Like hydroxy and alkoxy groups, the
ortho-/para-directing effect of amino groups is caused by the delocalization of a lone pair into the aromatic ring system. In other words, the
nitrogen can drop its lone pair into the benzene ring, creating a partial-positive charge on the nitrogen and a partial-negative charge on the ortho-
and para-carbons (hence their increased reactivity towards electrophiles).
When protonated, however, the nitrogen's lone pair can no longer do this, meaning the amino group can no longer donate electron density into the ring.
In fact, it will now even pull electron density out of the ring, as the nitrogen is already a highly electronegative atom to begin with,
which is now also electron-deficient on top of that. This is one of the reasons aniline nitrations give nearly 50% of the meta product.
Quote: Originally posted by CuReUS  | | I attributed this anomaly to the OH group of 2,4-dinitro-1-naphthol which activates the ring,but I may be wrong.Can someone explain ?
|
I don't think it's so much that the OH group activates the ring, but rather the fact that the nitro groups are specifically ortho and para to it. If
they were meta to the OH group, on the other hand, the reduction would likely either stop at the hydroxylamine stage, or at the very least take longer
to complete (probably requiring elevated temperatures as well).
What's likely going on in the case of 2,4-dinitro-1-naphthol is that the hydroxyl group is helping to facilitate the dehydration of the intermediate
hydroxylamine by forming an extremely reactive quinoneimine, which then gets rapidly reduced to the aminophenol. My guess is that the reduction goes
something like this:
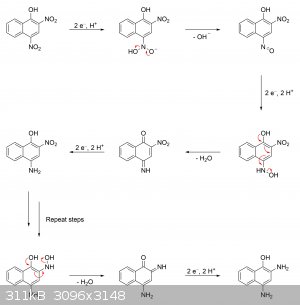
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  |
What's likely going on in the case of 2,4-dinitro-1-naphthol is that the hydroxyl group is helping to facilitate the dehydration of the intermediate
hydroxylamine by forming an extremely reactive quinoneimine, which then gets rapidly reduced to the aminophenol. |
that's a brilliant explanation darkstar  .So which procedure(chemplayer's vs
fieser's) has to be followed for reducing 2-methoxy-1,4-dinitrobenzene using dithionite ? .So which procedure(chemplayer's vs
fieser's) has to be followed for reducing 2-methoxy-1,4-dinitrobenzene using dithionite ?
in case the dithionite reduction fails,there is always Pd/C -http://pubs.rsc.org/en/content/articlelanding/2013/gc/c3gc37...
Boffis,don't forget to post a write-up once you finish synthesizing 2-methoxy-1,4-phenylenediamine 
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Can you get your hands on 2-aminoacetophenone? If so:
1. Acetylate the amino group to get 2-acetaminoacetophenone (1)
2. Nitrate (1) to get 2-acetamino-5-nitroacetophenone (2)
3. Baeyer-Villiger rxn (perborate?) on (2) to get 1-acetoxy-2-acetamino-5-nitrobenzene (3)
4. Remove acetoxy group of (3) and methylate phenol to get 1-methoxy-2-acetamino- 5-nitrobenzene (4)
The final steps from (4) are obvious though perhaps not without challenge.
AvB
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@ Darkstar, yes you are right. In that case do you think it would be better to try using HNO3 in methylene dichloride as the nitrating agent or
possibly HNO3 in acetic anhydride/GAA?
@AvBaeyer, 2-aminoacetophenone is tricky and pretty expensive. If I use my contacts to get this I might as well buy 2-methoxy-4-nitroaniline which is
one fifth of the price and can be converted into the target intermediate in one step  . .
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | | @ Darkstar, yes you are right. In that case do you think it would be better to try using HNO3 in methylene dichloride as the nitrating agent or
possibly HNO3 in acetic anhydride/GAA |
what's wrong with the french paper I linked above ? According to that(pg 271),you have to add the 3-nitroanisole in small portions to the nitric
acid.No solvent is required for the nitration.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by CuReUS  | Quote: Originally posted by Boffis  | | @ Darkstar, yes you are right. In that case do you think it would be better to try using HNO3 in methylene dichloride as the nitrating agent or
possibly HNO3 in acetic anhydride/GAA |
what's wrong with the french paper I linked above ? According to that(pg 271),you have to add the 3-nitroanisole in small portions to the nitric
acid.No solvent is required for the nitration. |
Your link gives only acces to the first page, the others are unreadable. 
Why would 3-nitroanisole (= 5-nitroanisole) form exclusively 1,4-dinitro-2-methoxybenzene (equal to 2,5-dinitroanisole) and not also
4,5-dinitroanisole and 2,3-dinitroanisole?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by CuReUS  | Quote: Originally posted by Boffis  | | @ Darkstar, yes you are right. In that case do you think it would be better to try using HNO3 in methylene dichloride as the nitrating agent or
possibly HNO3 in acetic anhydride/GAA |
what's wrong with the french paper I linked above ? According to that(pg 271),you have to add the 3-nitroanisole in small portions to the nitric
acid.No solvent is required for the nitration. |
Your link gives only acces to the first page, the others are unreadable. 
Why would 3-nitroanisole (= 5-nitroanisole) form exclusively 1,4-dinitro-2-methoxybenzene (equal to 2,5-dinitroanisole) and not also
4,5-dinitroanisole and 2,3-dinitroanisole? |
Thanks to CuReUS, I have the full article (18 pages).
It is in french, and I will try to translate it and post the translation here, but this can take a few days or weeks...end of the year is always busy
for everyone...you know Christmass, New-year, etc.
The author explains how he finds % of the various isomers in detail and how he isolates each of those.
Into the last page the author states exactly what I wrote...the 3 isomers are formed!
Thus from 3-nitroanisole (= 5-nitroanisole) nitration at 0°C by HNO3 (d=1,48; what is 89,07% by weight):
A) Isomer Epsilon/ 2,3-dinitroanisole (equal to 5,6-dinitroanisole) 51,2%
B) Isomer Gamma/ 2,5-dinitroanisole (equal to 3,6-dinitroanisole) 40,6%
C) Isomer Delta/ 4,5-dinitroanisole (equal to 3,4-dinitroanisole) 8,2%
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
PHILOU,could you show your calculations for converting the density to % weight for nitric acid.Because there is no corresponding % weight given for
nitric acid of density 1.48 at 0'C in this chart.The only %weight for d=1.48 is nitric acid at a concentration of 81% at 5'C
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by CuReUS  |
PHILOU,could you show your calculations for converting the density to % weight for nitric acid.Because there is no corresponding % weight given for
nitric acid of density 1.48 at 0'C in this chart.The only %weight for d=1.48 is nitric acid at a concentration of 81% at 5'C
|
No calculations.
The general specification of d into lab reports is at 20°C or at 25°C not at the temperature of the experiment! Of course at 5°C or 0°C the
density would be higher owing to contraction...
81% is at a density arround 1,455-1,456 at 20°C; 89% would be arround the 1,5 at 0-5°C
My value came from a table for HNO3 from Merck chemistry lab memento book p4...density at 20°C vs density of water at 4°C or
D20°4°
Since I had to take a picture of it and that I could in one shot take the page 4 and 5...I give you aswel those for H2SO4 and HCl.
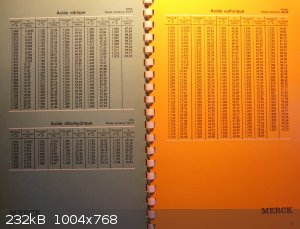
[Edited on 24-12-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi CeReUs, nothing is wrong with the French paper and I intend to try it but Christmas got in the way  . .
The fact that its in French isn't a problem either and it even describes the methylation of 3-nitrophenol to 3-nitroanisole with methyl iodide which
is the route I will ahve to take.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
From the density of HNO3 given,its obvious that fuming nitric acid has to be used.But I have a question that has been troubling me for the
past couple of days.Does red fuming nitric acid(RFNA) have to be used or only fuming nitric acid ? Does the dissolved NO2 make RFNA a more
stronger nitrating acid than just fuming nitric acid ? Btw fuming nitric acid is not same as white fuming nitric acid(WFNA) since the former has a
concentration of >90% whereas the latter is >99%
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I stumbled across this paper while looking for other papers in my archive and it opens up another route to my desired intermediate via
5-nitro-2-aminophenol which is here prepared from isatin, then methylated under basic conditions and finally reduced to 2,5-diamino-anisole aka
2-methoxy-1,4-phenylenediamine. For me isatin is a useful starting material because some years ago a guy was selling old 1 lb jars of the stuff so I
bought one. It is very old but appears perfectly OK. All I need now is time.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Boffis  | | I stumbled across this paper while looking for other papers in my archive and it opens up another route to my desired intermediate via
5-nitro-2-aminophenol which is here prepared from isatin, then methylated under basic conditions and finally reduced to 2,5-diamino-anisole aka
2-methoxy-1,4-phenylenediamine. For me isatin is a useful starting material because some years ago a guy was selling old 1 lb jars of the stuff so I
bought one. It is very old but appears perfectly OK. All I need now is time. |
There is a tread into the energetic section about diazodinitrophenols DDNP wherenitration of benzoxazolone is spoken about and this provide acces to the requested derivative of 5-nitro-2-aminophenol.
Rest of the tread is also interesting  . .
[Edited on 13-1-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 184
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  | | From the density of HNO3 given,its obvious that fuming nitric acid has to be used.But I have a question that has been troubling me for the
past couple of days.Does red fuming nitric acid(RFNA) have to be used or only fuming nitric acid ? Does the dissolved NO2 make RFNA a more
stronger nitrating acid than just fuming nitric acid ? Btw fuming nitric acid is not same as white fuming nitric acid(WFNA) since the former has a
concentration of >90% whereas the latter is >99% |
Red fuming nitric acid contains a lot of dissolved NO2, but that does not increase its nitrating properties. It can cause dangerous oxidation of
things, like the methyl group in the synthesis of TNT. In this case, red fuming nitric acid could likely be used, but the conversion from red fuming
to white fuming acid is a straightforward process. Air is bubbled through the red acid until the color clears up. Urea also reacts with dissolved
nitrogen dioxide, but it will decrease the concentration of the acid slightly, and if too much is added, urea nitrate may be formed. White fuming acid
is supposed to contain only <1% NO2, and <2% water.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by CuReUS  | | From the density of HNO3 given,its obvious that fuming nitric acid has to be used.But I have a question that has been troubling me for the
past couple of days.Does red fuming nitric acid(RFNA) have to be used or only fuming nitric acid ? Does the dissolved NO2 make RFNA a more
stronger nitrating acid than just fuming nitric acid ? Btw fuming nitric acid is not same as white fuming nitric acid(WFNA) since the former has a
concentration of >90% whereas the latter is >99% |
I think the main difference is that if the water content is too low, at warmer temperatures, the equilibrium will shift in favor of NO2, until the NO2
reaches a concentration of about 10%. You have to either inhibit or refrigerate WFNA to keep it from turning into RFNA. The reason that RFNA is only
90% concentration, is because most of the remaining 10% or so is NO2. I don't think it matters in many cases which one is used, because of the
aforementioned equilibrium. Though, if NO2/RFNA is the reactive agent, I'd imagine that those reactions would be done at higher temperatures, to
favor its formation, and reactions run at lower temperatures are likely to be run at those temperatures to inhibit NO2 formation.
If I remember correctly, NO2 is a stronger oxidizer, whereas HNO3 acts more like a typical acid, so which temperature you run it at might also depend
on what type of reaction you're trying to produce. If you have a choice, it's somewhat preferable to have WFNA though, because you can always make it
red just by warming it up.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
elemental P and melgar,thanks to you both.But I still have one doubt - is fuming nitric acid same as W/RFNA ?I don't think it is because the density
of fuming nitric acid is >90% whereas WFNA is >99%
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 184
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Fuming nitric acid is a general term given to nitric acid at a concentration high enough to produce nitrogen dioxide fumes on exposure to air. Red
fuming nitric acid is fuming nitric acid containing a lot of NO2, and white fuming is especially pure. Fuming is just the general term.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yeah. When they say that RFNA is 90% nitric acid, they're not counting the NO2, which is a nitric acid derivative, and can be converted to nitric
acid with hydrogen peroxide, among other things.
|
|
|