Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Imidazole Synthesis Route
Im trying to make Imidazole and i have come up with a synthetic route which i would like the forums opinion on. I hate to be the guy asking for advice
without any experiments but i am yet to make all the reagents. I am currently trying to make dioxirane (the first step).
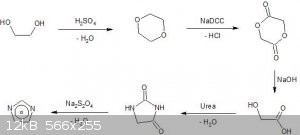
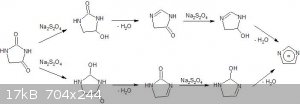
The first picture is the synthesis route and the second is the hydantoin reduction (Imidazole.jpg and Imidazole2.jpg respectivly).
1. The first step is the simple dehydration of ethylene glycol to dioxane catalysed by sulfuric acid.
PROCEDURE: Anhydrous ethylene glycol is distilled until foaming and release of sulfur oxides and the clear distillate separated by saturating the
solution with potassium carbonate and then dried with sodium hydroxide.
2. The second step is oxidation of Dioxane to Glycolide by SodiumDichloroisocyanuric acid. The reaction comes from https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/files/4593-DIRECT-CONVERSION-OF-ETHERS-TO-ESTERS-BY-TRICHLOROISOCYANURIC-ACIDbenzaldehyde-fr
om-benzyl-alcohol-and-tcca486a.pdf (not dioxirane specificaly)
PROCEDURE: Water is saturated with NADCC and Dioxane is periodically added. The resulting liquid is filtered and the flitrate is distilled with sodium
carbonate.
3. The third step is hydrolysis of Glycolide to Glycolic acid by sodium hydroxide. This reaction is not very complicated and my guess it is likly to
work. The question is if i could skip this step.
PROCEDURE: Aqeous sodium hydroxide is refluxed with Glycolide and the resulting solution is evaporated and recrystalized. (Not sure how to obtain pure
acid from sodium salt, maybe azeotropic distillation from sodium bisulfate and water?)
4. The fourth step is condensation of molten Urea and Glycolic acid (possibly glycolide). I think i read this in a paper but i can not seem find it.
PROCEDURE: Urea and Glycolic acid are fused at 200 C and the resulting slag is extracted with acetone and recrystalized.
5. The fifth and final step is the reduction of hydantoin by sodium dithionite and subsequent dehydration of the formed hemiaminal.
PROCEDURE: Sodium Dithionite, Sodium Bicarbonate, Hydantoin and a layer of Toluene is refluxed and Toluene layer separated and the evaporated to give
crude Imidazole.
As for uses for Imidazole there is ionic liquids, energetics and my obsession with it.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
To make hydantoin, you could probably just react glycine with potassium cyanate (https://en.wikipedia.org/wiki/Urech_hydantoin_synthesis ).
Glycine can be made from monochloroacetic acid.
[Edited on 12-13-2016 by Metacelsus]
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Potassium cyanate seems easy enough but glycine seems harder. I can not find any place that sells it as a food additive so i guess i would have to
make it.
Chloroacetic acid seems hard to make, atleast industrially with acetic anhydride catalyst, there is some otc chlorinations too but i dont have nearly
enough red phosphorous not to mention how dangerous those reactions are, atleast for a noob like me.
Gelatine contains 25-30 % glycine but i can not find a easy way to extract it, probably due to its low decomposition temprature.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Interesting. I was wondering what to do with this seemingly useless bottle of glycine that I bought from the health food store:
https://www.nowfoods.com/supplements/glycine-pure-powder
It was for another project, and ended up not being used at all.
I also have potassium carbonate, urea, a kiln, sodium bisulfite, zinc, and toluene. Dang it! You guys got me interested in yet another project that
I don't have time for!
Certainly post your results, Σldritch. I've been interested in making imidazole for a while, but have approached it from the direction of using
glyoxal, formaldehyde, and ammonia. The first two of those reagents aren't exactly over-the-counter.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by WGTR  | | I was wondering what to do with this seemingly useless bottle of glycine that I bought from the health food store. |
It's a nice chelating ligand.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
I was under the impression that dithionite could not reduce something like hydantoin, due to those carbonyls being amides and therefore caught up in
resonance with the nitrogen. Can you provide a reference that that works?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
You're probably right. Reaxys gives no results for hydantoin -> imidazole.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
convert your ethylene glycol to glyoxal -http://www.sciencemadness.org/talk/viewthread.php?tid=4425#p...
then do this-http://www.organic-chemistry.org/abstracts/lit2/593.shtm
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Thanks for all the replies, i think Cryolite is right about the hydantoin reduction too since one of the resonance structures seems to mess up the
reaction. Though i think it may still work but with crappy yields and a lot of crap as a byproduct.
Reduction in acidic media might work but the reactions i have seen which reduces amides to amines need PCl5 which defeats the purpose of this route.
Clemmensen reduction will not work since can not have a hemiaminal intermediate. (No hydroxyl group intermediate).
Also i was able to find a store that sells glycine 
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
http://www.orgsyn.org/Content/pdfs/procedures/CV3P0471.pdf
Degradation of histidine could also be of synthetic utility. TCCA in mild base should chew it up to (4-imidazolyl)acetic acid. You're on your own
after that.
Another approach might be condensation of ethylenediamine with chloroform or an orthoformate ester to 2-Imidazoline which can presumably be oxidized
to the target compound.
[Edited on 15-12-2016 by UC235]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
On the subject of imidazole syntheses does anyone think that this reaction might be possible:
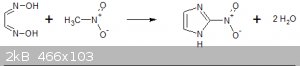
Basically it is the reaction of glyoxime and nitromethane. Under basic condition nitromethane condenses with all sorts of reagent; glyoxal,
formaldehyde, acrylonitrile, benzaldehyde...
but oximes? Maybe someone with a better grasp of theoretical chemistry than me can shed some light on the idea. Would basic condition be best?
|
|
|