Zool
Harmless

Posts: 18
Registered: 6-1-2017
Member Is Offline
Mood: No Mood
|
|
Inorganic synthesis - triphenyl phoshpine bis pyridine isothiocyanato copper I from copper II sulphate
Copper II vs Copper I (theory)
Copper I thiocyanate can be made with a very easy and very dangerous reaction at the same time . Copper I is a soft acid as it is a d10 metal.So
copper I should be a very stable oxidation state of copper since we listen many times that d5 and d10 electron configuration are more stable.So why
copper II is so much stable and this many times confuses people. There are many reasons why this happens but the most important (my opinion ) reason
is that everything is relative . In a gas phase really the Cu +1 oxidation state should be more stable because of the d10 electron configuration . But
Cu I is a soft acid and water is hard base . So in solvent water the Cu +1 is not the optimum since the complexation of Cu + 1 with water would have
as an effect a Hard-Soft complex and these are not prefered by nature the reason being that the one has the tension to form covalents bond while the
other has tension to form electrostatic bonds . Is like a bond between the sodium and carbon not really prefered . So metals are more complicated than
carbon and they can do all kind of things to get around not prefered situations. For example in a water environment with other hard ligands like
chloride ,water , hydroxide , carbonate , nitrate , anilline , ammonia , etc the copper forms much more stable compounds with these ligands and in a
hard solvent environment (water , ethanol , methanol) if he just become Copper +2 by losing one electron .Cu II is a hard acid and so it makes
electrostatic interactions more than covalent bonds . While Cu I make covalent bonds like carbon the Cu I uses his next 4s 4p empty orbitals and
hybridise them to give sp sp2 and sp3 hybrids depending on the number of ligands around it.Ligands give there HOMO electrons to the hybridised
orbitals of Cu I and make covalent bonds with copper I .
So in a synthetic project when you need a copper I compound as a starting material a good tactic is start from a water soluble Copper II compounds
like [Cu(H2O)4]SO4*H2O and dissolve the ligand you want the copper I to have along with the copper II compound in water (you can use
[Cu(H2O)4]SO4*H2O and CuCl2 ) or some alcohol (ethanol methanol - better use CuCl2) or even acetone .There if the ligand you put is soft when the
complex formation is happening there is formed a high energy hard-soft intermediate that some times you can stabilize and collect it.Most time if you
heat this intermediate up a disproportionation reaction happens where two of the intermediates react in a radical type reaction where the soft ligands
get oxidized and the copper II is reduced to copper I for both of the copper complexes . many times if the ligand that you choose to put can dimerise
it will dimerise to form stuff like (CN-CN if you put cyanide ligand) (NCS-SCS if you put thiocyanate ligand) and if it cannot form dimer like some
steric hindered phosphines the will get oxidized to phosphites ((R)3 )- P = O
Also Iodide ligand will form Iodine molecules I2) .
This work only if the ligand is soft if you want a intermediate or another ligand you cannot use this technique instead just put some thiosulphate or
sulphite and reduce it directly .
Copper is very flexible in hard solvent with hard ligands it form Copper II compounds in a variety of geometries (tetrahedral , octahedral ,distorted
octahedral , square planar) and colours (almost all spectrum) from the other hand in soft solvents (eg acetonitrile , pyridine ) with soft ligand (eg
I- , CN- , SCN - , phosphines) it forms copper I compounds with a lot of interesting structural chemistry from geometry point of view one molecule of
Cu I compound can be (linear (sp) , trigonal planar (sp)2 , tetrahedral (sp)3) like carbon! and from structural point of view the copper I complexes
form many times polymers or crazy structured clusters that are trimers and tetramers and for low number of ligands polymers .In this experiment I
started from copper II sulphate and and up in triphenyl phosphine bis pyridine isothiocyanato copper I . The method of how to do it half come from my
head (first pard synthesis of CuSCN) and the second come from nurdrage's video take a look if you haven't seen : https://www.youtube.com/watch?v=hPtCvReouCM )
part 1
Copper II sulphate to copper I thiocyanate
Use gloves eye shield do it outside or in a fumehood for no reason inside house because in this part some serious toxic stuff is produced like
hydrogen cyanide , thiocyanic acid and thiocyanogen . If you breath this stuff you die is simple so take caution .
put 20 mmol of CuSO4 anhydrous or hydrated it doesnt matter in 250 ml of water and stirr until you get a homogenous solution of copper II solvated [Cu
(H2O)6]^2+.
next make a solution of 40 mmol potassiun thiocyanate in minimum water ,the water will get colder since it is an endothermic solvation .
After you have two homogenous solutions mix these too an you get straight a black precipitate with not any interestin colour . This is our
intermediate trans-[Cu II (NCS)2 (H2O)2] of square planar geometry
The next step is very dangerous so use ventilation or do it outside for no reason dont do this inside a room with no ventilation.
Heat the water solution with the black precipitate without filtering out the precipitate . Slowly the black precipitate will change colour and become
a gray white precipitate what is happening now is the disproportionation reaction under here I show the mechanism
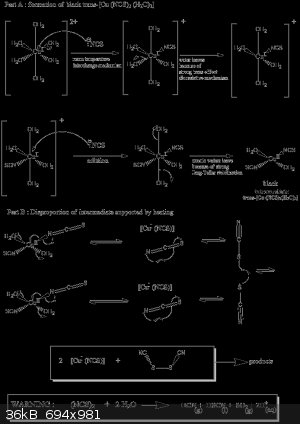
after you see no more black precipitate filter the white precipitate clean in with water and ethanol and make it dry with a vacuum pump on a filter.
Be carefull as tha filtrate and the solid contain some cyanide anions and HCN vapors . After take the gray white solid put it in a beaker and heat to
100 degrees C until it get completely dry and kind of creamy in colour. measure mas and calculate mole and yield for me it was > 80 %.
part 2
Now for the fun part
take 10 mmol of CuNCS 10 mmol of P(Ph)3 and 25 ml of pyridine heat the mixture to around 80 C and wait until everything is dissolved .The solution
will take a yellow colour . No heat the micture to 80 C for some 20 to 30 minutes for best results (higher yield and purer product) reflux 3 hours) .
Me I am impatient so after 20 minutes I take it off heat and close the top of the beaker with a petri dish. After I wrap the beaker with cotton and
after with aluminum foil to make it cool slowly.After around half hour big lamps of crystals formed . Decant properly the dirty pyridine and keep the
crystals . Clean the crystals with benzene or toluene and let them get dry . measure mass mole's and yield . Our yield was around 56 % .
The compound is strongly fluorescent and triboluminescent under I show the second reaction mechanism as well as photos of the compound and its
fluorescent property .
 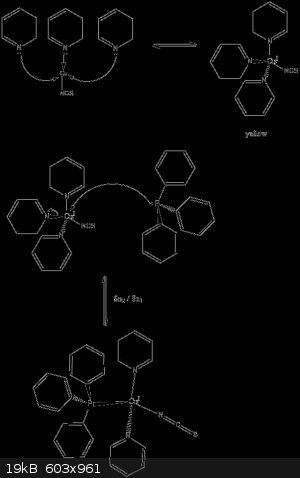  
[Edited on 7-2-2017 by Zool]
[Edited on 7-2-2017 by Zool]
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
The safer way to make CuSCN is to first add sodium metabisulfite, or sodium sulfite, to your solution of Cu(II), then add your SCN- solution. The
CuSCN precipitates as a tan/grey powder. The pre-reduction of Cu(II) avoids the formation of (SCN)2 (which I believe is formed from the reaction above
and hydrolyzes to HCN).
More details here:
http://www.sciencemadness.org/talk/viewthread.php?tid=29070
|
|
|
Zool
Harmless

Posts: 18
Registered: 6-1-2017
Member Is Offline
Mood: No Mood
|
|
True I mensioned that method , but at the moment I didn't have sulfite at the moment and I wanted to show an alternative method from the classic
preperation . Never the less as I said if you do the experiment outside or in a fumehood and with all the proper protecting equipment you are as safe
as doing chemistry with many other toxic stuff like hydrazine , azides , H2S etc
|
|
|
|