A Halogenated Substance
Hazard to Self
 
Posts: 68
Registered: 7-2-2017
Location: United States
Member Is Offline
Mood: Oxidizing due to extended exposure to oxygen
|
|
What did I make? (Experiment w/ Acetone, Sulfur, and NaOH)
Introduction (This is just backstory on how I came up with the idea; skippable):
Several days ago, I was flipping through some organic chemistry notes to try to come up with ideas for experimentation and I saw that before a
haloform reaction when sodium hydroxide reacts with a ketone to create an emolate, an alkene intermediate is formed. I decided that I wanted to try to
bind two emolates together using sulfur to create a sulfide.
I asked my chemistry teacher about this and he told me that it would likely not work as the sulfur would have to reduce something in order to oxidize.
I decided to at the very least try my idea small scale anyways.
Main Materials Used:
-1 40 ml. beaker
-1 50 ml. erlmyr. flask
-40-50 ml. cold water (to be added to flask as make-shift condenser)
-6.2 g. or 0.1 mol Acetone (Acetone was directly from a bottle of nail polish, ingredients listed as acetone and denatonijm benzoate only)
-1.6 g. or 0.05 mol Sulfur
-3.9 g. or 0.1 mol NaOH
-Stirring rod
-Hotplate
-Thermometer
-Safety glasses and gloves
Reaction Idea:
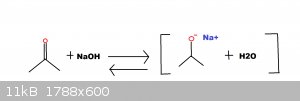
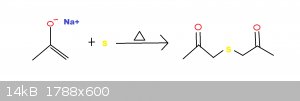
Procedure:
1)All materials gathered and weighed.
2)40-50 ml. water added to flask.
3)Added required amounts of acetone (6.2 g.), sodium hydroxide (3.9 g.), and sulfur (1.6 g.) accordingly.
4)Heat contents of beaker on hotplate; position flask on top of beaker to condense escaping acetone.Moniter temperature occasionally.
Observation:
-From 20 to 30 degrees celsius, the mixture steadily began to take on a weak brown-grey color.
-From 40 to 50 degrees celsius, the mixture began boiling and took on a brown color. A slight sulfurous smell was noticed.
-Stopped heating around 52 degrees celsius. The mixture began to take a dark brown-red color.
5)Allowed to cool. The mixture remained a dark brown-red color and gradually becoming thicker and thicker. The consistency eventually became that of a
thick paste. The mass of the contents in the beaker upon cooling turned to be 6.8 grams.
6)Mixed a little bit of the sample with water, created a deep yellow solution.
Pictures:

    
So does anyone have a different idea of what I did? I have just done this experiment this night and I am not 100% completely sure of the products.
However, further testing and refining will be done later to verify what I did. I would enjoy feedback or suggestions.
Thank you!
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
acetone + sulfur dissolved in aqueous base has been discussed here a few times, but I don't know that a solid conclusion has been reached yet. You
might look at this post (and the thread containing it) for more info:
http://www.sciencemadness.org/talk/viewthread.php?tid=66390#...
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
j_sum1
Administrator
       
Posts: 6225
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I don't think anything much happened with the acetone: at least not in the direction that you were hoping.
Sulfur dissolves in strong sodium hydroxide solutions to form sodium polysulfide which is a rich yellow-orange colour.
As the name indicates, this substance is polymeric. It surprises me little that you ended up with a dark, tarry vile-smelling goop.
But good experimentation nonetheless. Thanks for sharing and thanks for taking the time to both explain yourself and provide pictures.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Before you try explaining the results, check to make sure your equations are balanced.
Anyway, I know from personal experience that alkyne anions will react with elemental sulfur to form thio-ynolates, which can then be trapped with
electrophiles. This is the closest analog I know of to your proposed reaction. I do not think a similar reaction would work with an enolate and
sulfur. However, if you really want to test it, I would suggest using a polar aprotic solvent and strong base (like LDA) to quantitatively form the
enolate. Then you can add the sulfur and see what happens. This might be somewhat inaccessible for the amateur, however.
|
|
|