| Pages:
1
2 |
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Preparation of Chrome Yellow and Chrome Orange
Introduction:
Chrome Yellow (lead chromate, PbCrO4) and Chrome Orange (basic lead chromate, PbCrO4: PbO) are two compounds most notably used as paint pigments both
for artistic and industrial use. Their use has declined somewhat with the advent of cadmium pigments and more so with the increasing use of organic
pigments and dyes for similar purposes. They are, however, still an easily accessible pair to synthesize for the home chemist.
Warning! This experiment requires both the use and disposal of compounds containing lead and hexavalent chromium. Gloves and a clean,
spacious work environment are highly recommended. Hexavalent chromium is disposed of relatively easily using bisulfite, thiosulfate, or ethanol to
convert it to the less-worrisome trivalent chromium. Significant amounts of lead-contaminated paper and water are produced during filtration steps,
however, and these need to be dealt with in a more professional manner. Please be a responsible chemist and dispose of any lead waste responsibly and
legally.
Reagents used:
- lead nitrate (homemade from lead sinkers)
- potassium chromate (homemade from pottery-grade potassium dichromate)
- potassium hydroxide (food-grade, Duda Diesel); sodium hydroxide has also been used and works just as well
Not required, but highly recommended:
- nitric acid (homemade); a few drops are used to eliminate insoluble basic nitrates of lead
- sodium metabisulfite (stump remover); used to dispose of hexavalent chromium waste
Experimental:
Preparation of chrome yellow via the classic metathesis reaction
To start, two solutions are made. First, 8.00 grams of lead(II) nitrate are weighed out and dissolved in 100mL of warm water. A second solution,
containing 8.2 grams of potassium chromate, is prepared using 20mL of water. I used a large stoichiometric excess of potassium chromate for two
reasons: 1) my potassium chromate had recently been crystallized from solution and was still wet, and 2) it is imperative that an excess is used to
prevent water-soluble lead compounds from remaining in the filtrate.
 
The two solutions were heated on a hot plate, but carefully so as not to have them boiling; fine droplets of lead nitrate solution are not something I
want being splashed into the air I breathe. Even with all the lead nitrate dissolved, the solution was still cloudy due to the presence of insoluble
basic nitrates. A few drops of nitric acid were added and the solution clarified at once. An excess of acid is not problematic; in fact, the reaction
seems to work essentially the same whether chromate or dichromate is used. With everything dissolved, the potassium chromate solution was poured into
the lead nitrate solution, immediately giving a fine, richly-yellow precipitate of lead(II) chromate; our chrome yellow.
 
The precipitate was easily filtered out using a coffee filter and washed liberally with cold water. I chose to dry it on the paper using a food dehydrator I purchased online; it has a huge capacity provided by a rack of trays and dries things under air at about 60-70 degrees C. I
highly recommend them over throwing them in your home oven; just don’t use the same one for your beef jerky. After about half an hour the chrome
yellow was removed and taken to the next step.
  
Preparation of chrome orange from chrome yellow
Chrome orange is traditionally prepared by leaching chrome yellow with a strong alkali; converting some of the surface of the pigment particles to
lead(II) oxide in the process. This was easier said than done, as I’ll explain later.
3 grams of the dry lead(II) chromate produced earlier were measured out and added to 15mL of boiling water to form a slurry. The amount of water
should be kept as low as possible but enough should be used that the slurry is thin and can be stirred quite rapidly. Before the next step is carried
out, a few things need to be ready and at hand, as it must be carried out very quickly. With a clean filtration setup and a beaker containing 20mL of
ice-cold water close at hand, the potassium hydroxide flakes were added all in one go to the already boiling slurry of lead chromate with rapid
stirring by hand. Immediately the mixture turned a striking orange color.
 
Before the color could change any further, the contents of the beaker were gravity filtered and repeatedly washed with small portions of cold water.
Prolonged exposure to caustic solutions can ruin the orange color of the chrome orange and leach it to a dingy brown color. As can be seen from the
filter paper below, the supernatant is bright yellow with sodium chromate as a result of the leeching. After the precipitate was cleaned, it was dried
in the same way as before, and stored alongside the chrome yellow and a vial of chrome orange previously prepared in several batches, as seen at the
bottom of the write-up.
 
Discussion
This preparation is one I can now consistently perform successfully, but the intial trial-and-error process of preparing a satisfactory orange color
in the past was rather tricky. Adding the potassium hydroxide to a solution of potassium chromate to basify it and then adding the lead nitrate
solution as vague procedures suggested only produced light brown or dull yellow-orange precipitates. Adding lead(II) chromate to solutions of sodium
hydroxide at varying concentrations and temperatures can work to some degree, but the products are inconsistent, ranging from light brown to a deep
rust color almost randomly. This is the only tried and true method I've been able to prepare it by, and unfortunately, like the benzaldehyde prep I
recently posted, this is just something I came across on my own rather than from an established, well-written synthesis to use as a reference.

[Edited on 2-17-2017 by Amos]
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Online
Mood: desperate for shade
|
|
Nice work, I like the colors. I did not know that the cloudiness in PbNO3 solutions was from the basic nitrate and how to clear it up. Thanks for the
tip.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Might there be some antimony nitrate in your lead nitrate?
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
When I initially dissolved the lead sinkers, I had a small amount of white powder leftover; I've been told this is actually antimony pentoxide, which
forms as a result of oxidation of the antimony by the nitric acid. The pentoxide is insoluble in nitric acid and is easily filtered off. I don't
actually see any sources that confirm the existence of antimony nitrates at any oxidation state.
[Edited on 2-17-2017 by Amos]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Amos  |
When I initially dissolved the lead sinkers, I had a small amount of white powder leftover; I've been told this is actually antimony pentoxide, which
forms as a result of oxidation of the antimony by the nitric acid. The pentoxide is insoluble in nitric acid and is easily filtered off. I don't
actually see any sources that confirm the existence of antimony nitrates at any oxidation state.
[Edited on 2-17-2017 by Amos] |
Fascinating. It looks as though antimony nitrate doesn't ordinarily form when antimony is dissolved in nitric acid: https://books.google.com/books?id=vVhpurkfeN4C&pg=PA193#...
Well done with the chrome yellow and chrome orange.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Wonderful bright colours Amos, congratulations.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Looks beautiful, Amos. And a lovely write-up too.
How safe are these to actually use as pigments? They seem like the kind of thing that people have been trying to phase out for the last few decades.
(My guess is that if you don't plan on licking your oil paintings you are probably ok.)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I suggest you to work also on lead dichromate PbCr2O7...also quite orange and obtained simply by mixing solutions of a soluble dichromate salt like Na
or K with a solution of a soluble lead salt (nitrate, acetate, ...)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by PHILOU Zrealone  | | I suggest you to work also on lead dichromate PbCr2O7...also quite orange and obtained simply by mixing solutions of a soluble dichromate salt like Na
or K with a solution of a soluble lead salt (nitrate, acetate, ...) |
Have you tried this yourself and noticed this? I've done the very same and only got the same yellow color from my precipitate. Although, given the
instability of Chrome Orange to acid, it could be that my lead nitrate solution was too acidic from the get-go.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Beautiful colors!
You mentioned disposal was important, but didn't elaborate on how you did it (for Pb anyway). How did you dispose of your lead- and chromium-bearing
solutions?
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by MrHomeScientist  | Beautiful colors!
You mentioned disposal was important, but didn't elaborate on how you did it (for Pb anyway). How did you dispose of your lead- and chromium-bearing
solutions? |
Ah, I suppose this is rather important. All chromium waste in my lab gets reduced to Cr(III) and precipitated as the hydroxide, which I use for
further experimentation, since I actually find chromium's chemistry more fascinating at lower oxidation states.
Lead is trickier. During the leeching process, not just chromate, but also sodium plumbite or other soluble anions are produced. This is noticed when
the filtrate is acidified; the plumbite is unstable at the lower pH and a precipitate of yellow lead(II) chromate is once again seen. So it is
important that the solution is not assumed relatively lead-free at high pH just because you don't see any turbidity.
I still have my liquid lead waste which I will be treating with sodium sulfide solution to precipitate any last bit of lead hiding out as the sulfide;
with luck I might have enough to find a use for. But I'm not pulling out the sodium sulfide until I begin my next project (hint: it's red!) as it's
quite stinky.
Any lead-containing filter papers go into my heavy metal waste receptacle, which when full will go to my local Haz Bin, which accepts these sorts of
things from individuals. Most cities should have a convenient equivalent. Thanks for asking!
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Thanks for answering! To be honest I hear a lot of people (especially on YouTube) talking about 'proper disposal' but rarely do they elaborate on it.
I think disposal is very important to cover when reporting an experiment, so others can try it safely. Especially when heavy metals are involved!
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by j_sum1  | Looks beautiful, Amos. And a lovely write-up too.
How safe are these to actually use as pigments? They seem like the kind of thing that people have been trying to phase out for the last few decades.
(My guess is that if you don't plan on licking your oil paintings you are probably ok.) |
Accidentally skipped over your question; the answer is I'm not sure. Neither of these is the most chemically stable; chrome yellow is somewhat
sensitive to base as you can see, leeching to give alkali chromates; not too big a deal. Acids attack the chrome orange, though, leading to lead
salts. Neither is too great to breathe or eat. But since my destination for these is oil paint, which locks up the dust and protects the pigment
particles from chemical weathering (paintings from the 1800s using them are generally still very bright), I think it should be pretty safe in this
regard. Really old school buses and airplanes likely still have chrome yellow paint on the outside, which I imagine is more dangerous as it can flake
off the metal underneath. It has since been replaced with azo pigments for the most part on vehicles. Oh, and thanks! Glad you like the write-up!
[Edited on 2-17-2017 by Amos]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Amos  | Quote: Originally posted by PHILOU Zrealone  | | I suggest you to work also on lead dichromate PbCr2O7...also quite orange and obtained simply by mixing solutions of a soluble dichromate salt like Na
or K with a solution of a soluble lead salt (nitrate, acetate, ...) |
Have you tried this yourself and noticed this? I've done the very same and only got the same yellow color from my precipitate. Although, given the
instability of Chrome Orange to acid, it could be that my lead nitrate solution was too acidic from the get-go. |
I indeed did it...the dichromate of Pb(II) is deep orange while Pb(II) chromate is yellow...both unsoluble precipitates...I did this 25 years ago out
of curiosity...for pigments...with saturated solutions of Pb(NO3)2, sodium chromate and sodium dichromate.
Silver chromate is also nice ....deep red...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
The good news is that any painting with it won't mold, mildew or get pests eating them for long. I have noticed that older white lead paints don't
mold or mildew in bathrooms, which is the one good things about them. Newer latex paints seem to mold quite easily unless you add anti-mildew
agents, which are often other nasty chemicals, those used to include tin salts, but those have also been phased out like lead, chrome, and many
cadmium salts. Hard to find many heavy metals that you can put in your pigments now.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Funnily enough Dr. Bob, it turns out that making my own paint pigments is actually cheaper than going out and buying paint already made in tubes. So
whether or not they're phased out doesn't bother me!
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report on making chrome yellow (lead chromate) and chrome orange (basic lead chromate) following (more or less) the procedure on Prepchem:
https://prepchem.com/synthesis-of-basic-lead-chromate/
Chrome yellow:
- 9g potassium dichromate dissolved in 100g water in beaker, reddish solution. Had to be heated a bit to get all to dissolve.
- 25g of lead acetate dissolved in 150g water in beaker, clear solution.

- Add the potassium dichromate solution slowly to the lead acetate solution while under magnetic stirring.
- Immediate yellow suspension, keep stirring 15 minutes. Very fine solid, not settling out easily when stirring is stopped.

- Vacuum filter. Some of the yellow ppt going through the filter paper and frit, and it is so fine that it made a foamy mass in the Erlenmeyer flask
and got sucked out the side port! I had to add another Erlenmeyer flask in line to prevent the lead chromate going into the pump.

- Rinse remainder in the funnel with water. Weight of wet recovered remainder was 25.3g. Lovely pure yellow color.

- Place in desiccator over NaOH and under vacuum to dry; with the hope that the vacuum would delay the natural change to the more basic orange lead
chromate.
- Remove from desiccator after 24 hours. Appears dry, weight reduced to 15g, but started to go orange on the outer layers of the chunks.

- Transfer to sealed bottle and shake bottle in an attempt to pulverise it. This hid most of the small bit of orange, but it is so fine that it sticks
to the sides of the bottle.

Chrome orange:
- 19g lead acetate in 200ml water
- 4.5g potassium dichromate in 60ml water
- 6.5g sodium hydroxide in 60ml water
- Add the potassium dichromate to the lead acetate, getting the yellow suspension as before.
- Add the sodium hydroxide.
- Stir and heat to boil. The procedure says to keep hot until there is no further color change. The color of my solution changed to red-orange in a
matter of minutes, and the color change seemed to complete long before it got near boiling. See below color of the solution some 5 minutes after
adding the sodium hydroxide.

- Keep near boil for an hour and 15 minutes while stirring. Final color below.

- Transfer to 2L beaker and wash 3 times with 500ml water by decantation.
- Vacuum filter. Wet remainder = 32g. Product appears much more coarse than the lead chromate, filtrate was clear.

Stopped for the night.
- Next day, dry on steam bath.
- First dry (3hrs) weight reduced from 32g to 15,1g
- Second dry (3 hrs) 15.1g to 12.1g.
- Third dry (2 hrs) 12.1g to 12.1g: as dry as its is going to get on the steam bath.
- Drying seemed to push the color more towards orange.
- Final product = 12g of orange-red (or is this brick red?) powder.

I have since found more procedures, I may have another go one day to try and get it more red.

|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
My chrome orange

|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
lead dichromate?
I wonder what happens when you keep the conditions acid when combining lead nitrate with potassium dichromate solution. I don't see how that could
lead to the formation of the basic lead chromate known as chrome orange. The result is however a compound intermediate in colour between chrome yellow
and chrome orange. Here's my test with lead nitrate in excess, pH ~ 1.
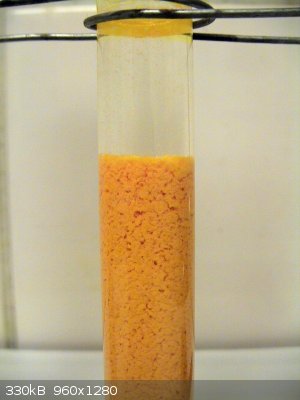
I cannot find any serious information about the existence of lead dichromate, but could this be it? It does not seem to have a CAS number, at least
not on ChemicalBook or PubChem, for what it's worth.
Besides, I also tried barium chloride in acid solution with potassium dichromate, but no precipitate was formed.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
In theory - why not? Your precipitate should be lead dichromate.
You can try analyse your precipitate - chromium iodometricaly, lead by chelatometry (pH 3-5, xylenol orange as indicator).
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I've had a handful of people tell me that you can obtain an orange lead dichromate by mixing those solutions, but I've never seen any evidence of the
veracity. Just "it was a long time ago, I don't have pictures".
Twice I have mixed solutions of crystal clear lead nitrate (always with a drop or two of nitric acid added to swing the equilibrium away from
hydrolysis) and sodium dichromate with each other and both times I was greeted with the same exact color as when using chromate, even at high
concentrations of both reagents.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
What about CrO3.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
What about it?
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
One shouldn't expect alkali salts and acid to always be the same as the acid free of other substances, especially when a precipitate is involved,
should they?
Like I wouldn't really expect "treating" (as Mellor says) to actually mean refluxing for several hours. (hint) Or maybe chemistry isn't that easy and
we can't just mix chemicals up and everything works out just like on paper.
[Edited on 3-4-2021 by S.C. Wack]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by S.C. Wack  | One shouldn't expect alkali salts and acid to always be the same as the acid free of other substances, especially when a precipitate is involved,
should they? (...)
[Edited on 3-4-2021 by S.C. Wack] |
Not always, but it often is the case. Also, for iodides, substances like potassium iodoplumbates exist, but alkali chromatoplumbates known?
Anyway, the possibility of alkali dichromatoplumbates exists, so I put it to the test.
Quote: Originally posted by Bezaleel  | | Besides, I also tried barium chloride in acid solution with potassium dichromate, but no precipitate was formed. |
After adding a bit of ammonia solution, pale yellow BaCrO4 was obtained. The solution was removed with a pipette, washed twice and dried. (The photo
below shows the dried PbCr2O7 as initially obtained, and the BaCrO4.)

0.557g of BaCrO4 was obtained. To the fine, dry powder 0.25ml of 44.1% H2SO4 was added (an appr. 1:0.8 ratio of CrO4(2-) to SO4(2-)) and 10ml of
water. An orange solution was obtained with the typical dichromate colour. pH ~ 0 with a test strip.
The clear solution was sucked up with a pipette and tested for left over sulphate. Addition of a few drops to a Ba-acetate solution of pH ~5 yielded a
pale yellow precipitate, but the amount of precipitate formed looked more than the amount of possible sulphate present could explain. A solution of
Ba(OH)2.8H2O in HNO3, still acid, did not produce any precipitate when the orange solution of CrO3 was added, so I assumed it to be sulphate free
(BaSO4 precipitates in acid solution containing Ba2+ ions).
Addition of a solution of PbNO3 (pH ~4) to the orange solution gave copious amounts of deep yellow precipitate, just a s before.

After standing, a dark yellow precipitate was obtained, as in the initial experiment.

|
|
|
| Pages:
1
2 |