MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Anti Static Pyro Compositions
Hello All,
I have been doing extensive research on pyrotechnic compositions and their sensitivity to static electricity... and have not been satisfied with any
potential solutions.
Obviously mixtures with metal particles are more static sensitive, such as 70/30 flash. And the smaller the metal particles the more sensitive to
static (I presume this is because a smaller particle will melt and vaporizes with a smaller energy input such as static). In regards to flash, I have
also heard it is static sensitive because the oxide layer is non conductive, and the inside Al is... essentially forming a capacitor.
So... lets take 70/30 (2-5 micron flake Al as an example... if I don't want to use a larger Al size because performance will drop... what are my
options?
1) Mixing in a non conductive clay, although I hear this reduces performance. Has anyone tried this?
2) Perhaps coating the Al powder with a thin (micron to nanometer) coat of wax or animal fat, sterin? Again, has anyone tried this?
3) I can't find any information on this in regards to pyrotechnics but I know copper nano particles (5-7nm) are used in making anti static plastics.
If this were mixed in with the flash powder wouldn't it create a Gaussian cage?? surly at 5-7nm all of the particles would be evenly coated and
surrounded. Perhaps mill the Al with copper nano particles?
That is all of my ideas..
Bert your response is mandatory because you have experience in all of these things, or you can always point me in the right direction...
I don't care if the solution is expensive... lets partake in some thought experiments, or actual experiments!!
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I wonder if adding some graphite powder would help dissipate static charges. It might be worth a try.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
I am pretty sure coating with wax or polymer works, i wonder what requirements you need from your mixtures since wax coating usually reduces the burn
rate.
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Are there any polymers that work particularly well for Al that you know of?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
From what I remember form my 20 year ago chemical engineering lessons:
The oxide layer is part of the problem but also the bad conductivity of other ingredients (amorphous Carbon, Sulfur, wax coating)...
If you understand wel what is static electricity and a capacitor...you clearly get the feeling that the core of the problem is the charge separation
from insulating materials (by friction, swirling, flowing) and eventually the mutual charge induction of metallic material.
It is known that into hydrophobic non conductive solvents flowing into metallic pipes or into plastic ones (that are not grounded to the soil)...that
you can have hurge electrostatic potential and discharge...like thunder bolts...
The static electricity will also be reduced if metallic particles get in touch with each others (thus forming kind a continuous phase entraping non
conductive nuggets --> Gaussian cage); or following the reverse idea a lot of insulating material to keep the metallic particles to be able to
spark between each others...thus increasing the spark gap.
Wax or polymer may thus not be the smartest to use...and the waxy coating treatment of Al powder (reducing its sensitivity to moist air) asmuch as the
Al2O3 or Al(OH)O layer are insulating the metallic core of the particles.
In fact the best option would be to render the powder conductive so that all charges can exit or fade...but increasing interparticulate conductivity
also means a storage concern...because of the inherent reactivity of Al powder...or a severe reduction of initiability (typical example is moisture)
The use of Cu powder will not play any role... because it will play the same role as the Al...
A Gaussian cage needs continuous contact media...sothat the charges can repel each other to the extremal external surface...the inside is thus
chargeless...so you need continuous wires, grids or planes or volumes eventually with voïds...
Also the surface of the metal plays a role..."the power of nails" (I have found no translation of this into English...but the Lightning rod is based
on that principle...and generates a ionised wind); so when there is a sharp edge usually this means charges gathers there and the electrostatic
potential is localy increased...air passing by (or other materials can be ionised) and thus the material discharges by gaseous convection...This can
be seen into the dark by some glowing of the pin point (St-Elmo's fire)...so if the metallic conductor is smooth, polished, with moderate curves
(ideally perfectly sperical), it will hold its charge longer and be less prone to easily dicharge and sparks.
Absolutely not an easy subject...also very depending onto atmospheric conditions (heat/moisture).
[Edited on 27-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Philou,
Thank you for your reply... I understand what you are saying but have a few questions.
If the best thing is for the metallic particles to touch each other than why do compositions become more static sensitive when nano particles are
induced... as they completely coat a 120 micron oxidizer particle?
How would nano copper or silver not help with this, especially if they were 5-7nm, they would coat everything, and silver, being a noble metal will
not oxidize easily?
So for this flash powder, would it be better to use spherical Al 500nm instead of the 2-5 micron flake?... but then it will become more impact
sensitive... ahhh!
[Edited on 27-2-2017 by MineMan]
[Edited on 27-2-2017 by MineMan]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by MineMan  | Philou,
Thank you for your reply... I understand what you are saying but have a few questions.
If the best thing is for the metallic particles to touch each other than why do compositions become more static sensitive when nano particles are
induced... as they completely coat a 120 micron oxidizer particle?
How would nano copper or silver not help with this, especially if they were 5-7nm, they would coat everything, and silver, being a noble metal will
not oxidize easily?
So for this flash powder, would it be better to use spherical Al 500nm instead of the 2-5 micron flake?... but then it will become more impact
sensitive... ahhh!
|
Usullay they don't coat efficiently...have you made a simultation or a calculation to say this? Can you show me the calculation to see if it is
correct?
Apparently...it is hard to understand or visualize (again linked to the "pin point power"): if you have two different particle size of the same
material brought at the same electric potential...the radius reduction of the particle reduces the overal electric charge of the particle
propotionnally...and at the same distance (bigger than R) the electric field is also lower
So if R = 2*r
$$q R = V × 4 π ε 0 R {\displaystyle q_{R}=V\times 4\pi \varepsilon _{0}R} {\displaystyle q_{R}=V\times 4\pi \varepsilon _{0}R}$$
$${\displaystyle q_{r}=V\times 4\pi \varepsilon _{0}r={\frac {V\times 4\pi \varepsilon _{0}R}{2}}={\frac {q_{R}}{2}}}$$
$${\displaystyle E_{R}={\frac {V\times R}{d^{2}}}}$$
$${\displaystyle E_{r}={\frac {V\times r}{d^{2}}}={\frac {V\times R/2}{d^{2}}}={\frac {1}{2}}{\frac {V\times R}{d^{2}}}={\frac {E_{R}}{2}}}$$
so at the distance d (what is further than R itself twice as big as r)...the electric field of the particle r is reduced by a factor 2 vs particle R.
But if you go in close contact with the external layer for the tinier particle r you can go much closer than into the R case since R is unpenetrable
solid and charges are onto the outer shell/surface.
So
$${\displaystyle E_{R}={\frac {V\times R}{R^{2}}}}$$
$${\displaystyle E_{r}={\frac {V\times r}{r^{2}}}={\frac {V\times R/2}{(R/2)^{2}}}={\frac {V\times R/2}{R^{2}/4}}=2{\frac {V\times
R}{R^{2}}}=2E_{R}}$$
And in fine, we observe that the electric field in close viccinity to the surface of the tinier particle will be twice bigger than the one in close
viccinity of the larger one.
By extension...if the radius is n times smaller...the electric field at the surface will be n times bigger.
It comes from the fact that the sum of the charges q set on a spherical surface submitted to an electric potential V is increasing linearly with the
increasing radius while the electric field decreases at the same time as a function of 1/d².
If you consider a pin point as a very small diameter half sphere (what is the case if you look at it under strong magnification)...the electric field
obtained at that point has a value that tends to the infinity...and this very high field will contribute to air ionisation, and electric arc
triggering.
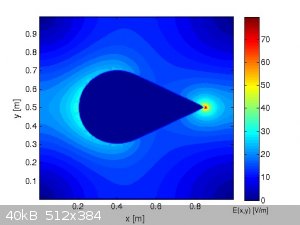
Last thing to consider for R vs r (=1/2*R)...if at the surface of r you have an electric field twice bigger...for the same weight of metal (at the
same density) you have also the same volume...
So
VR = 4/3*π*R3
and
Vr = 4/3*π*r3
thus
Vr = 4/3*π*(R/2)3 = 1/23*4/3*π*R3 = 1/8*VR
You thus have potentially 8 times more electric field per equivalent weight without counting the already double value...
In the case of a reduction of factor n (R=n*r) you thus have for each particule r an electric field n*ER bigger but also n3
times more particles than into the R case...and this makes a serious increase of n3*n*ER = n4*ER
So reducing the radius by a factor 2 increases the risk of static by 16...I let you imagine what happens when passing from micro to nano sized
metallic powder....
Silver and copper are near "noble metals" they don't oxidise easily from air or oxygen...but they are oxidised by trace poluants from air (ammonia,
CO2, H2SO4, H2S)...silver has the bad habit to turn dark grey/black upon time...but this will normally not lead to connectivity troubles only a %
reduction (how much I don't know).
In theory from all what is explained here above...mixes with larger spherical particles will be less prone to static...and flakes will increase static
because of their sharp edges at the end of the plannar surface acting like a pin point all arround the flake.
[Edited on 28-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
phlogiston
International Hazard
    
Posts: 1375
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Speculating here, but for large sparks, it is also a requirement that the charge can move quickly towards the point of discharge.
That is why you can get long bright sparks from the metal sphere from a van der graaf accelerator, but not from the bottom plastic roller even though
the amount of charge stored in each should be roughly the same.
Translating that to your question, at higher concentrations of metal powder, bigger networks of interconnected particles can form that can transport a
larger amount of the charge stored in the powder quickly to the location where it is dissipated in the form of a spark.
Maybe adding a poorly conducting powder (graphite?) will help, by dissipating the energy gradually along the way as ohmic heating rather than as a
localised, high-energy spark?
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by phlogiston  | Speculating here, but for large sparks, it is also a requirement that the charge can move quickly towards the point of discharge.
That is why you can get long bright sparks from the metal sphere from a van der graaf accelerator, but not from the bottom plastic roller even though
the amount of charge stored in each should be roughly the same.
Translating that to your question, at higher concentrations of metal powder, bigger networks of interconnected particles can form that can transport a
larger amount of the charge stored in the powder quickly to the location where it is dissipated in the form of a spark.
Maybe adding a poorly conducting powder (graphite?) will help, by dissipating the energy gradually along the way as ohmic heating rather than as a
localised, high-energy spark? |
Graphite comes into line of my proposal to increase the conductivity of the media...
This can be done for example:
-by moisturizing (but is detrimental to sensitivity and initiation)...
-by increasing the amount of metallic powder (but then detrimental to OB)...
Graphite spray is known to be used to render certains surfaces a-static...it is used for example when making O2/Acetylen baloons because of the
inherent risk of detonation induced by static on such a sensitive and powerful gas mix.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Yes, my experiments are showing that graphite may be valuable.... I just wish there was an easy non expensive way to test static sensitivity... The
only way I know of is using a BBQ Piezo spark..
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by MineMan  | | Yes, my experiments are showing that graphite may be valuable.... I just wish there was an easy non expensive way to test static sensitivity... The
only way I know of is using a BBQ Piezo spark.. |
You need a device that can variate the electric field/charge and the distance --> a "sparker gapper" if I may name it so...
The gap/distance when the spark is triggered defines the charge of the metallic ball and the energy provided by the spark.
To make one maybe get inspiration from a Van der Graaf device or a static charge holding bottle.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
Commercial smokeless powder reduces static sensitivity by coating the grains with graphite. This increases their conductivity (you can measure it
with an simple ohm meter through lightly compressed powder in the 1000 ohm range)
The electrical power is P=I^2*R reducing R reduces the heating in the powder so the electrical power ends up being dissipated "somewhere else" in the
circuit, probably in the air above the powder.
|
|
|