Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
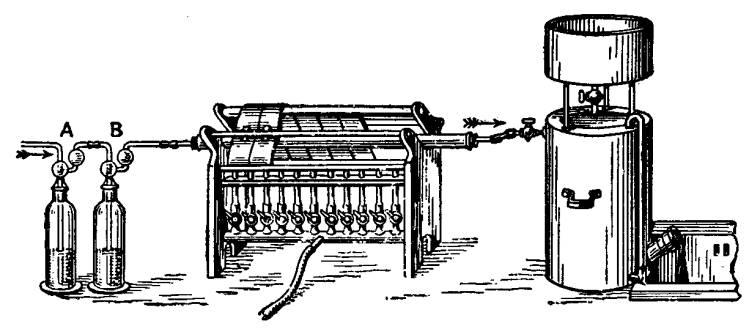
N2 - nitrogen from air
| Quote: | Nitrogen is easily obtained from air by removing the admixed carbon dioxide and oxygen. Air freed from carbon dioxide in a wash bottle of sodium
hydroxide, A, and from moisture by passage through sulphuric acid, B, is then passed through a red-hot tube containing copper turnings. The copper
removes the oxygen and forms cupric oxide
2Cu+02=2CuO
The nitrogen passes on to be collected in a gas jar, or gasholder, etc. In the diagram, the air is supposed to be drawn over the copper, the gasholder
being filled with nitrogen. If the gasholder were placed at the end A, and air forced along the tubes, the nitrogen gas could be collected in gas
jars. Cold boiled water should be, used in the gasholder so as to lessen the risk of contamination owing to the presence of oxygen dissolved in
ordinary water. The process of oxidation of course ceases when all the copper is oxidized. If the air, before passing over the red-hot copper, be led
through an aq. soln. of ammonia, the ammonia reduces the copper oxide as fast as it is formed:
2Cu+02+nN2=2CuO+nN2
and
3CuO+2NH3 =3Cu+3H2O+N2
Any excess of ammonia can be removed by passing the gas from the copper tube through a soln. of sulphuric acid before it is collected in the
gasholder. |
Ripped from the HIVE posted by lugh (not the Lugh registered here).
Thats to important in my eyes to be scrupelous as it provides a easy and cheap source of inert gas - one of the safety related features missing for
most of us I believe. How much reactions can be made safer and cleaner and how many even get possible by such a device! Gasphase reactions where a
carriergas is needed for example, and and and......
The preferable setup seems to be:
- a airpump (aquarium will do fine or slave operated bellows)
- through a washbottle with conc. NaOH
- through a washbottle with ammoniawater, to
- a nozzle (to keep direction of flow, works like a valve)
- into a red hot coppertube filled with copper filings, scrubbers, wire... (preferable heated electrically)
- a SS coil to cool down (plain iron is ok, watercooled if necessary)
- through the final conc. H2SO4 scrubber removing H2O and NH3.
Also this may sound like a lot of effort I don´t see it this way as the apparatus consists of old bottles mainly and can be arranged very space
preserving. The only costfactor may be the airpump if no slaves available. The rest is to salvage with ease.

|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
When using ammonia, you'll have H2 contamination. Where does that go to?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
?
It´s well balanced, isn´t it?
| Quote: |
3CuO+2NH3 =3Cu+3H2O+N2
|
Could you elaborate a little more please?
I see a postulation and miss the arguments backing it up.
???????????????????????????????????
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Ammonia is in excess, it will decompose to nitrogen and hydrogen significantly.
Hydrogen will also form from partial oxidation of ammonia in the low oxygen part of the system.
Hot ammonia is rather reactive to most transition metals, I cant remember offhand if what forms is the nitride of the metal, and hydrogen gas, or the
hydride of the metal and nitrogen gas but I'm sure the ammonia is decomposed and this is a big problem with pipes carrying hot (eg red hot)
ammonia.
I think with regard to chemistry, the basic copper reduction of air is a fine method if pure nitrogen is the goal and no other sources exist. Its a
lot of effort to go just for an inert atmosphere though, and far from being cheep, you are having to maintaina sizable furnace at red heat for the
entire duration you need nitrogen.
Most people dont have the ability to be able to test the resulting gas easily for purity, you can snuff a candle, but the gas might contain enough
oxygen to completely destroy an organo lithium reaction for example.
The furnace assembly is a major fire hazard unless its totally insulated, a fire hazard that doesnt need to be present if heating mantles are used for
heating flasks.
For a typical small scale reaction, a bubble or two of nitrogen per second hardly seem to justify such an elaberate, and potentially unreliable gas
generator compaired with using welders nitrogen for example.
[Edited on 28-5-2003 by Marvin]
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
Ammonia burning catalysis
Platinum is used to burn NH<sub>3</sub> in O<sub>2</sub> to NO and H<sub>2</sub>O whereas copper can be used to
catalyze the reactants to N<sub>2</sub> and H<sub>2</sub>O. I don't think it would matter if it was a copper oxide or
just the metallic form, it should do the same thing just with the copper oxide doing both the catalysis and the providing of the oxygen.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
out of reach
Nitrogen bottles are out of reach for most here. (I would prefer Argon anyways). Same for highly sensibel organolithium reactions. But Grignards would
benefit and many other reactions which are possible to carry out without inert gas flushing but run more smoothly with.
I agree that a way should be developed to detect excess oxygen and to shut down the process or at least warn the experimentor.
Marvin your argumentation leads only to the final "buy everything readymade from Sigma-Aldrich or Fluka". Can´t be the last of wisdom I
believe.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
As ever Organikum, you have a talent for taking things to their illogical conclusion. I'm not suggesting we should buy everything, but I'm
also not suggesting we should make everything we are ever going to need starting from hydrogen and using a home fusion reactor powered with microwave
ovens. (Sticks tongue out in the general direction of the Czech Republic)  . .
Welding is done all over the planet, I suspect its just a matter of finding somewhere local to you.
In the event nitrogen refilling is not economical where you are or too far to go, you could always use something else like butane for grignard. Since
the flow rate is low the hazards arnt much greater than for the boiling ether usually involved anyway. Its cheap, its pure, its dry, its stored with
high density and with regard to grignard and many similar reactions, its essentially inert.
Making nitrogen the hard way maybe fractionally cheeper, but if its only a tiny percentage of the cost of the reagents to use bottled nitrogen anyway,
the saving is unlikley to justify the effort.
The loss in most grignard reactions due to oxygen is negligable compaired to wurtz reactions which arnt avoidable, and water reactions which though
standard lab practice virtually negates this, will be a large threat to home grignard working at all.
Considering this, I dont see the point in the extra effort thats more likley to result in people giving up on an experiment than achieving higher
yeilds.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
I read the thread again twice
and found that nowhere I was advising or recommending anyone to build and run such a nitrogen generator. Nowhere, never.
Of course, inert gases botteled fresh from LINDE or else are to prefer no doubt. The decision what to do is in everyones own hands - but a real
decision needs alternatives to choose from. This is one. Not less not more.
The device described was widely used in laboratories and small industrial factories until bottled nitrogen got affordable as a result of the first
world war. This is not Captain Nemos Nautilus - this was reality.
- These devices worked well and will do this today also - except the laws of thermodynamics have changed.
- Gas from bottles is more safe and more easy. Nobody is advised to build or run such a apparatus - but who wants to can do so now. There is a choice.
- Who is thinking on building other hot tube devices now or later for ketene or alcohol dehydrogenations/oxidations or thermolysis of any kind, who
wants to go this way, he is advised, strongly advised to start with this generator as it contains all essential parts of a hot tube and enables one to
gain practical experiences at low risk.
I have the opinion the members of this board may decide by themselfs whats useful or best for them and if in doubt they may ask someone they believe
to have the necessary knowledge.
Marvin, you are a teacher, right? 
Your advise to run Grignards under butane shows that you have by no way safety in mind but only some "I am right and you are wrong".
Don´t speak safety when you mean feeding your ego.
[Edited on 30-5-2003 by Organikum]
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
It is well known that different countries regulate different chemicals. For instance, I cannot legally buy formaldehyde, nitrite or chlorate here (
and it is not because I haven't looked for them, they are banned to all but those with the proper licenses ), but I have no trouble getting HNO3,
H2SO4, KMnO4 or sulfur.
Therefore it is potentially valuable to know how to produce any given chemical.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
"Your advise to run Grignards under butane shows that you have by no way safety in mind"
Organikum, what is wrong with a Grignard under butane? It's not as if anyone would be stupid enough to vent the excess butane into a sealed lab
full of naked flames. Just vent it out the window through a hose. You're using ether anyway, which is just as much of a fire risk.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
You are right Nick
This was to be seen in relation to the topic of this thread. A generator for inert gas was taken down with arguments which proofed wrong and then
without any arguments just for the "I am right" nonsense.
How dangerous is a Grignard in relation to the this divice anyways? Ten, hundertfold? And it doesnt get safer by the the use of butane for sure. It
would get safer by the use of nitrogen.
It´s the doublethink I dislike and the attitude.
If one wants to do some experiments in chemistry without an Sigma-Aldrich account tube furnances are one of the few means left to produce compounds of
interest. They are easier to build and run as electrolytic cells (which are true industrial devices and a pain in the ass for the amateur IMHO) and
more versatile. They are if handled with common sense and regarding the usual laboratory rules very safe also - this is based in the working principle
what includes that there is actually only a tiny amount at a time processed. And this process takes place in a metaltube. If the whole content of the
tube decomposes you will have a "puff" and a short flame on the ends. If I try to chlorinate some toluene by the Loomis patent with bleach
for to get to benzaldehyde - I can make very sudden the experience how high the content of energy in half an liter toluene actually is - enough for a
hell of flames. Same can happen by an electrolytic process - by any process where the whole soup is processed in one pot.
But by no way I will tell: Hey! You must try this! But if somebody is already on the way I want to have him a look at this.
This nitrogen generator is a perfect start - useful, working and safe as amateur experimentalism can be. (at least the interesting part of it). I
expect no enthusiasm but I am loosing patience on this. Here is the synthesis of high explosives discussed which are regarded as to unsafe for any
industrial or even military use - but tube furnances are industrial and the devils?
Reality check please!
Perhaps some have a problem with me not coming to chemistry from the interest on explosives and weapons. They may behold this problem for themselfes
as I don´t want it.
A atmosphere of help, constructive critics and support would be nice. What about using ones knowledge in this sense? And just because one feels pissed
he doesn´t have to piss on others, capiche?
end rant.
ORG 
btw. the only way to get rid of me is Polverone throwing me out. Just to kill some possibly existing illusions. 
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
last not least
The nitrogen generator was originally posted as told by lugh at the HIVE.
lugh is moderator there and has the necessary education and is known to be always correct on the facts to assure that chemistry and setup are ok.
The generator was worth to be posted here I believe and it was also for to assure myself as I started getting those mean doubts on myself (more than
the usual not so mean doubts), started feeling unsure and unsecure. And so proof was needed and achieved. The proof that it´s not on me spacing out
but on some meanies doing critics for the critics sake.
Puhh! 
I feel better now. 
Hey! Meanies! You´ll get ulcer from this, you know?
(and the penis shrinks, but thats another story..)
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Rather unsurprisingly Organikum, I have a few points to make. Unlike yours however, I will try to restrict myself to objective commentary about what
youve raised, and the science.
| Quote: |
I read the thread again twice
and found that nowhere I was advising or recommending anyone to build and run such a nitrogen generator. Nowhere, never.
|
You have said,
| Quote: |
it provides a easy and cheap source of inert gas - one of the safety related features missing for most of us I believe. How much reactions can be
made safer and cleaner and how many even get possible by such a device!
|
This constitutes an endorcement, even if starting the thread in the first place was not considered endorcement enough. I am commenting on the safety,
reliability and cost effectiveness of the idea, and nowhere have *I* said it should *not* be done. Ive even said, that specifically to make pure
nitrogen it is a good method. If in difference to your posts you do not endorce the method, you shouldnt be bothered by any valid critisisms.
| Quote: |
The decision what to do is in everyones own hands - but a real decision needs alternatives to choose from.
|
Then discuss the *specific* pros and cons of this and the alternatives.
| Quote: |
These devices worked well and will do this today also
|
They worked in the past and they will still work today. They wernt easy to use in the past, they wernt low maintinance when they were working, they
wernt reliable, but with some effort they could be assembled by anyone interested, and there was nothing else feasable. Now we have pure nitrogen
available everywhere and to everyone at small to industrial quantities and industrial prices.
| Quote: |
Who is thinking on building other hot tube devices now or later for ketene or alcohol dehydrogenations/oxidations or thermolysis of any kind, who
wants to go this way, he is advised, strongly advised to start with this generator as it contains all essential parts of a hot tube and enables one to
gain practical experiences at low risk.
|
You are on a crusade to get people to use 'industrial' chemistry at home. We know.
| Quote: |
I have the opinion the members of this board may decide by themselfs whats useful or best for them and if in doubt they may ask someone they believe
to have the necessary knowledge.
|
If by this you mean, 'decide themselves without any general public discussion' then you apear to have missed the whole point behind posting
in a forum. We dont wait for people to ask for advice, we post what we know on a subject in order to get as much information on that subject as
possible in one place. People will decide for themselves, they always do, but they should be alowed to do so with as much information from multiple
sources rather than liking what may be a terrible idea and having to ask a single person for guidance.
| Quote: |
Your advise to run Grignards under butane shows that you have by no way safety in mind but only some "I am right and you are wrong".
|
Why do you think butane is so unsafe? Is it becuase nitrogen is 'non flammable' and butane is 'flammable'? This maybe true for
the pure gas going in, but this is in some doubt for the exit gasses you need to get rid of. Assuming a typical grignard setup, the boiling ether is
under reflux using tapwater cooled condensors, say 10-12C for the tapwater, 16-17C effective temp for the exit gas which is fine for maintaining
reflux. But when you pass a carrier gas through the system, the exit gas will be saturated with ether, at that temp thats about 500mbar of vapour.
It nolonger matters which of the two gasses is used to trickle through the system becuase the exit gas will be flammable anyway!
You can buy explosion proof mantles (mantles which when on will not ignite flammable gas mixtures), and anyone with safety in mind would be using one
for grignard. Other methods are suitable which are cheaper such as an electrically heated oilbaths. Having a furnace in close proximity to, or even
in the same room, as the boiling ether is not safe. It wouldnt be alowed in a proper lab, and it shouldnt be done at home, there are enough
alternatives. Compairing butane to ether:- ether has wider flammability mixtures, it has a higher flame velocity, using butane as a carrier gas for
it does not increase the hazards much.
| Quote: |
How dangerous is a Grignard in relation to the this divice anyways?
|
You are asking the wrong question. What you should be asking is 'How much more/less dangerous would a grignard be using this device instead of
the alternatives'. Nitrogen bottles and regulators are fairly pricey but the refilling is cheap, they are reliable, widely available and safe.
Butane camping stoves and refils are cheep, reliable, easy to handle and for a flammable gas the flammability limits are fairly narrow, the flame
velocity is low. The largest hazard here for general use is keeping the liquid butane cannisters away from the source of heat, this is standard lab
practice and with mantles is not difficult anyway.
| Quote: |
tube furnances are one of the few means left to produce compounds of interest.....regarding the usual laboratory rules very safe also
|
Tube furnaces require a lot more than the usual lab rules. You need much more information just to be safe, than to succeed safely in a standard lab
synthesis, Better fume extraction with outside being frequently not enough, to be able to prepare catalysts for the majority of useful reactions and
to control many more variables to get good yeilds which will be different for every homebuilt setup. They are a very powerful tools, but they are not
a definite synthesis, so much as an experiment to find the optimum conditions, coupled with the advantage of wanting the products.
| Quote: |
Here is the synthesis of high explosives discussed which are regarded as to unsafe for any industrial or even military use - but tube furnances are
industrial and the devils?
|
Other people are discussing explosives thought to be too unsafe to be in general use. You are discussing tube furnace setups that are considered too
unsafe for general use. There is less of an intrinsic difference than you imply.
As ever, risk must be considered relative to the alternatives. Heating a grignard reaction with a naked flame is universally held to be unsafe. Its
not becuase the first time someone tries it the flask will explode and kill them, its becuase its so much more risky than the accepted methods of
doing it, and becuase of the consequences when something happens.
| Quote: |
.....there and has the necessary education and is known to be always correct.....
|
Noone is always correct. This logic is unacceptable.
| Quote: |
A atmosphere of help, constructive critics and support would be nice.
|
You have this, but whenever someone points out a problem, you take it like a personal insult.
| Quote: |
It´s the doublethink I dislike and the attitude.
|
There is no doublethink here and the only attitude is what you brought with you.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
I restrain from doing the same
And I won´t go nitpicking and wordscrewing point for point through Marvins post. I will insult him later in persona as he deserves this, thats all.
"People may decide by themselves" here was exactly repeated what I told just in other words. And this as common point maybe the subject:
Who reads this thread may decide by himselve. 
On the facts there was nothing new, the argument "you can buy nitrogen in bottles" was already told before and answered by Microtek. Nothing
to add.
No arguments. Some vague dark words on special conditions and dangerous catalysts which would be needed to run a tube furnace - nothing factual only
the invocation of ghosts. My EtOH to acetaldehyde tube runs with plain copper as catalyst. Was hard and dangerous to prepare oh yeah! Worse is the
ketene tube where the catalyst was impossible to prepare - there is none needed. Whatta nonsense.
A look at the setup:
- We boil a liquid.
(this may be regarded as common practice in chemistry I believe)
- We vent the vapours through a hot tube
(this is the special part)
- We condense vapours from the tube and collect them
(Common practice too)
Ok, the special part why is this so undoable, dangerous, industrial?
In reality this hot tube has an important advantage over the usual one-pot reactions: There is at a time only a very small amount of reactands
processed. The actual reaction chamber (tube) is quite small from volume and contains catalyst. The reactands are in gasphase.
Also if the complete content of the reaction chamber decomposes all of a sudden - this isn´t much. And so there isn´t much to happen
(a state of the art setup regarding safety is understood - should be always I want to say).
If a one pot reaction runs away - this can get other dimensions as everyone here will have encountered already.
promised insult:
Marvin, you are a liar:
| Quote: | Unlike yours however, I will try to restrict myself to objective commentary
|
Unlike you who named me a crusader I had restricted to attack you in persona before. All what you bitch about is caused by the fact that you took it
as yours. It wasn´t written this way. I wrote my post before intentionally unpersonal in style for to keep emotions low. Doesn´t help a lot with
princesses...
And it is a lowlevel cheapo rethoric trick, old as stone but works often. Boring in my eyes and negotiating the intelligence of the reader.
I prefer it straight: As promised I insulted Marvin
(by naming him a liar, whats actual the truth as proofen)
Couldn´t I have left out this revanche? Sorry - no, my gloriole is in the wash.
final, reality check:
The ART and SCIENCE of AMATEUR EXPERIMENTALISM.
Here we are. Thats my subject. Thats what I like. My attitude is NOT telling people what to do and what not to do. Anarchist? Yes earlier I was, much
earlier but meanwhile I degenerated to a fucking liberal. Thus comes by getting older, can´t help it.

|
|
|
Iv4
Hazard to Others
  
Posts: 312
Registered: 28-5-2003
Member Is Offline
Mood: No Mood
|
|
This is the second proccess described by my chemistry book and it does'nt really sound bad to me.If you think getting copper red hot is a problem
try having to vaporise CuCL.I've done my share of laser research and can honestly say it's not very hard to createa furnace that only gets
copper red hot.
I'm thinking of a ceramic furnace(off hand I dont have the exact formula but it's mainly cement with sand glass and plaster in it)with two
or three IR heating elemnts.The best ofcourse would be a quartz tube but then again you can buy a lot of nitroegn for it's cost...
My chemistry book simpy says through sodium hydroxide and into the furnace.Come to think of it the carbon dioxide is'nt THAT big a problem so it
could just be an open furnace.
The first reaction for making nitrogen was ammoniam nitrite and sodium hydroxide heated together.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Copper deposited
on Celite (Kieselguhr) or pumice would be superior to a plain tube of course. The more on surface area makes it. But with the ammonia reducing the
oxide back to the metal this looses on importance - what speaks against a plain tube is the danger of oxygen coming through by the straight flow. So
a filled tube with deposited copper ona carrier as told is favorable.
Tube heating is easily applied by NiCr-wire from a salvaged hairdryer or electric heater which gets fixed in furnace cement/waterglass (silicates).
Rockwool will give cheap and easy insulation.
As tv4 told - a tube in whole is easily made from furnace cement - some glassfiberfabric will give the mechanical strength wanted and needed.
No rocket science - basic knowledge on electrics is necessary so - as very often in amateur experimentalism.
Difficulties are in the joints , in special the joint between boiler and tube is critical.
|
|
|
Iv4
Hazard to Others
  
Posts: 312
Registered: 28-5-2003
Member Is Offline
Mood: No Mood
|
|
It seems to me that it's better to forego the ammonia untill effeciancy is unacceptable and then disconected the collecter and run ammonia
through the tube.This elimantes the problem of contaminating the ammonia but will have to monitered.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
contaminating what with what?
Excess ammonia will be eliminated by a H2SO4 (conc.) washbottle which scavenges also all H2O present.
But wait.
This maybe an excellent idea btw! I will have to think this over before I am sure but a system with two or more tubes whereby one tube is working
whilst the other gets reduced by ammonia or else - hm.
where is my muse? Ah - here she comes!
Inspiration please! 
|
|
|
Iv4
Hazard to Others
  
Posts: 312
Registered: 28-5-2003
Member Is Offline
Mood: No Mood
|
|
Sorry I meant that the h2s04 could be excluded so it would be little more than the furnace alone(considering co2 is'nt usuallyin very high
concentration it might be neccesry to use hydroxide/water even!).I'm a bit drunk or I'd think something up mechanicaly.
|
|
|
alchemie
Harmless

Posts: 36
Registered: 4-6-2003
Member Is Offline
Mood: variational
|
|
Although the contruction of a tube furnace is an appealing endeavour and, as Organikum says, it´s use is not restricted to N2 production, there is an
esier way to remove the O2 from air using Cu, and, get this, at room temperature.
here´s the method:
Two flasks, both with openings on the bottom, are connected with a ruber tube(something similar to a push-pull device,), and these are filled to 3/4
with pieces of copper(turnings or whatever). One ot these flasks is closed with a stopper bearing a tube with a stopcock, and the other one is filled
with ammonia solution. a part of the ammonia passes into the bottom of the other flask and that is shaken to wet the copperand the apparatus is
allowed to stand. After 24 hours have passed the O2 inside the flask has been absorved, forming "copper- ammonia nitrite" and one has to
only open the stopcock to obtain the N2, wich is displaced by the ammoniacal solution of the other flask. Passing the gas through dilute H2SO4 removes
the NH3 gas. Or concentrated H2SO4 could be used to, simultaneously, dry the N2 and catch the NH3.
I hope this is intelligible enough, if not I can try to explain better and/or get some drawings.
I´ve also read somewhere a method to do this as a continuous operation, but can´t remember where, I´ll try to look it up.
NaSO3(sodum sulfite) also absorves atmosferic O2, forming Na2SO4. IF bisulphite also works(it should) then this would be a much better alternative to
all this copper-ammonia-furnace-or-not-crazy-ideas.
[Edited on 8-6-2003 by alchemie]
|
|
|
Iv4
Hazard to Others
  
Posts: 312
Registered: 28-5-2003
Member Is Offline
Mood: No Mood
|
|
Not to insult you or so but the furnace method seems easier and it would give larger amounts of N2.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Improvements
alchemie, this sounds highly inefficient to me - alone as nitrogen gets used up for the nitrite.
But perhaps this nitrite is a compound worth to look at, as easily prepared as seen?
To the hot tube: It´s understood that the higher the surface area the higher the working area of the tube. Duration and volume per timeunit can be
multiplied by using a tube filled with copper finely deposited on pumice or celite (kieselguhr). The copper may be precipated on celite from a
solution of copper nitrate by adding NAOH solution. The celite and the metallic copper deposited should be of the same weight.
(adapted from the patent: GB687745, Aldehydes/Ketones production)
This catalyst can be used in every tube able to withstand the heat, just beware of possible negative catalytic effects of other metals and use plain
copper or ceramics. One to five complete oxidation/reduction cycles before use boost performance which is minimum 5x better as a plain tube, 10x to
50x is standard, more possible.
And, hey! You can make aldehydes and ketones with this tube. Already with the plain coppertube but don´t do it, use pumice or celite, deposit some
metals of your choice and most aldehydes and many ketones are yours.
And thus is only a start. 
Other as most historic crusaders I don´t bring black death and gods word (in my variation of course), but some aldehydes and ketones and a world of
synthesis to discover.
Orgy off, waving a glowing tube, reciting loud passages out of ancient patents....
   
can you see it Polverone? 
[Edited on 9-6-2003 by Organikum]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
| Quote: |
And I won´t go nitpicking and wordscrewing point for point through Marvins post. I will insult him later in persona as he deserves this, thats all.
|
Thankyou for that objective assessment. I bring up points becuase I think they need to be raised. If noone brings qualified objections, you'll
forgive me for assuming they are accurate.
| Quote: |
On the facts there was nothing new, the argument "you can buy nitrogen in bottles" was already told before and answered by Microtek. Nothing
to add.
|
I was doing a comparason of the pros and cons of several methods, I could hardly have left out the most common and widely applicable method.
Microtek's point that is always useful to know how to make chemicals from what is around is a very valid one. This however has no bearing on
what would be the best choice to get nitrogen for use in a home lab.
| Quote: |
No arguments. Some dark words on special conditions and dangerous catalysts...
|
Absolutly, no arguments in that at all. Maybe you'd prefer to call them 'discussions, technical points, safety concerns' as
apropriate?
I have suggested that the catalysts are often difficult to prepare, and the products/byproducts are often very dangerous. What did you choose as an
example for an easy and safe reaction? The production of ketene. A toxic gas which in the literiture is frequently compaired to phosgene, used as a
war gas. Youve made my point better than I did.
| Quote: |
Ok, the special part why is this so undoable, dangerous, industrial?
|
Unsafe in certain circumstances, highly unsafe in some suggested situations certainly. Undoable? You are reading non existant objections, or perhaps
somewhere I said that a heated tube full of copper was a physically impossible feat of human engineering? Industrial? I'm not sure the word
even has any real meaning in this thread outside of sheer scale, and that is completely in control of the experimenter. If you feel there is a stigma
associated with the word 'industrial' maybe its because those reactions used in industry tend to be those that give the lowest cost product
in enviroments that can be engineered to be vastly better controlled and contained than is feasable in a lab.
As far as safety goes, the nitrogen generator is as safe as a hot tube reaction is likley to get. Its only when you start daisy chaining equipment
that the safety problems start to multiply.
| Quote: |
...if the complete content of the reaction chamber decomposes all of a sudden....
|
But this isnt likley to happen to any wet reaction that a lab chemist would be call 'safe' either. A wet reaction can run away, but this
should not be a major hazard. Your argument is nullified by a lack of a comparason with an equivalent wet reaction and by the generic differences
between wet and hot tube reactions. Any real advantages will be valid only for specific goals and in specific amounts. Production of aldehydes would
be a good example, but its a trade of beween setting up the equipment, and the price of the reagents but even here you can get nitre from about $2
US/kilogram.
| Quote: |
I prefer it straight: As promised I insulted Marvin
(by naming him a liar, whats actual the truth as proofen)
|
You keep saying these things as if youve allready shown me to be wrong. Yet you havnt countered any of my specific technical points nor have you
suggested how to improve safety matters or aspects of feasability in specific setups. Instead you acuse me of lying and feeding my ego, argueing only
the general merits of tube furnaces. How do you resolve these points?
| Quote: |
final, reality check:
|
You feel the need to check reality, thats fine, I feel the need to check the chemistry.
(Other issues).
Making 'ammonium nitrite', ie mixing an ammonium compound and a nitrite to produce nitrogen gas is a good method for pure nitrogen. A bit
of a waste of nitrite though just to provide an inert gas.
Sulphites as I understand it are very slow in absorbing oxygen, and the exit gas would need scrubbing with NaOH to remove sulphur dioxide.
Absorbing oxygen using copper/ammonia solution is potentially useful but I would think rather slow for flowing gas production. As I understand it the
reaction is more or less just catalysed oxidation of the copper. A copper II salt is added to ammonia producing very dark blue solution containing
[Cu(NH3)4]2+. This is added to copper metal and this produces colourless complexed copper I ions. The complexed copper I reacts with oxygen to
produce copper II, and the cycle repeats. The majority produces would be expected to be a solution of copper salt, and copper hydroxide, in ammonia.
I would expect nitrite to occur only in trace amounts, if at all. Nitrite and ammonia/ammonium react to form nitrogen gas anyway under these
condions. This reaction is potentially useful, as the colour change indicated the presence of oxygen. If the nitrogen from the furnace was allowed
to bubble into the copper I/ammonia solution, a blue colour would indicate the nitrogen contained noticable oxygen, though this might not be fast
enough to actually remove all the oxygen from it. Since the copper II ammonia complex is very strongly absorbing, this should be quite a sensitive
reaction.
Adding sodium hydroxide to copper nitrate solution doesnt produce metallic copper. You get a gelatinous light blue (usually) hydroxide precipitated
which turns black as it dehydrates. The example in the patent reduces the catalyst with a stream of hydrogen gas. The situation in the nitrogen
generator is reducing, but the conditions arnt the same (patent seems to indicate reduction is in the cold!), so it might be less active.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Adding NaOH to coppernitrate doesn´t precipitate metalic copper. Thats right of course. I apologize for being sloppy in my use of words. The
"metallic copper" referred to the weight in relation to the substrate.
Cu(OH)2 is precipitated which gets dehydrated by heating to CuO and this is reduced to Cu by hydrogen or ammonia. The activity comes by the higher
surface area of thus precipitated copper so the remark on lowered activity is nonsense as it lacks all understanding.
You are right and I am wrong.
[Edited on 23-6-2003 by vulture]
|
|
|
Z8320
Harmless

Posts: 7
Registered: 13-12-2009
Member Is Offline
Mood: No Mood
|
|
Sorry to dredge up an old topic; has anyone done this and determined its efficacy using any kind of qualitative or quantitative testing?
My other thought was that it may be unnecessary to actually build a tube furnace or obtain copper powder for the process; it appears to me that you'd
be better off using the copper itself as the heating element. It may need to be rewired periodically, but you could pack copper wool into a glass
tube and pass current through the copper. As contacts for the copper wool, you could have metal tubing on either side of the tube to be heated. I'll
have an oxygen sensor in the next couple weeks and I'll try this out. In general, I suspect this will be much, much more efficient than using a tube
furnace, and it may actually make this a fairly low power device.
I'll post the setup with data later.
|
|
|