AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
5-Oxo-2-tetrahydrofurancarboxylic acid (butyrolacton-5-carboxylic acid) from glutamic acid
Introduction
I anticipated using 5-oxo-2-tetrahydrofurancarboxylic acid as an intermediate in a multi-step synthesis of 4-penten-1-ol via 4-pentenoic acid. The
conversion of glutamic acid into 5-oxo-2-tetrahydrofurancarboxylic acid is well established [1-4] and all the reagents required for the conversion are
readily available. The proposed use of 5-oxo-2-tetrahydrofurancarboxylic acid as an intermediate for 4-penten-1-ol has since been abandoned but I
thought I would present my experimental results for the synthesis of this compound. The general scheme is shown below.
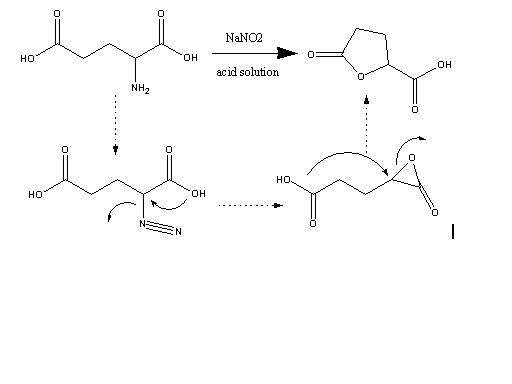
Reaction of an alpha-amino acid with nitrous acid gives the diazo acid which reacts with the alpha-carboxylate to give the transient 3-membered
lactone which then goes on to react with a nucleophile which in this case is the terminal carboxylic acid group of glutamic acid or alternatively with
water to give the hydroxy acid (not shown). The hydroxy acid cyclizes to the lactone during work up. The general mechanism as illustrated is well
established but I will not go into this further at this time. It is also well established that the reaction proceeds with net retention of
stereochemistry at what was the amine bearing carbon atom.
Typically, the above reaction is run at cold temperatures and often for an extended period of time [1-4]. The reaction solution is acidified with
hydrochloric acid or sulfuric acid. The work up involves a very tedious and time consuming distillation of a large volume of water under reduced
pressure followed by extraction of the pot concentrate with a suitable solvent.
While searching for physical and chemical data related to the desired lactone product, I came across a patent which claimed that the conversion of
glutamic acid to the desired lactone could be accomplished in a short time at elevated temperature [5]. Though this looked a bit too good to be true I
decided to try this method first. However, the tedious reaction work up was still a challenge.
I also wanted to eliminate the use of sulfuric acid. I chose to use sodium bisulfate as the acidifying agent instead. This has the advantage that it
can be accurately weighed so that the amount of acid added can be more carefully controlled.
In order to reduce the number of experimental variables, I ran the reaction as described in the aforementioned patent substituting sodium bisulfate
for the sulfuric acid and concentrated the reaction solution at water aspirator pressure. This is described in Experiment 1.
Experiment 2 describes a re-run of Experiment 1 except that the reaction was concentrated by simply boiling at atmospheric pressure.
Finally, while doing more literature searching I came across a Japanese patent abstract [6] which claimed that the lactone could be readily extracted
if the reaction mixture was saturated with an inorganic salt. This variation is described in Experiment 3.
Results and Discussion
First, the use of sodium bisulfate as the acid source worked quite well. The material used was that widely sold for swimming pool acidification. The
label assay was assumed to state the true purity of the sodium bisulfate.
Experiment 1 was run according to the patent procedure [5]. Reduced pressure concentration of the reaction solution took several hours but did yield a
semi-solid pot residue consisting of hydrated sodium sulfate/bisulfate and the lactone product. Extraction of the residue with ethyl acetate provided
a crystalline product in 75% yield.
In experiment 2, initially run as described in the patent, the reaction mixture was concentrated by gentle boiling with stirring. The same
crystalline product was obtained in 54% total yield.
Finally, in experiment 3 the reaction was carried out as in experiments 1 and 2. The reaction solution after addition of ammonium sulfate and
extraction with ethyl acetate gave a crystalline product in 75% overall yield. In this experiment, it should be possible to use far fewer extractions
than I did by employing larger amounts of ethyl acetate for each extraction.
The purity of the product could not be accurately determined. The product has a yellow color but when pure should be a white solid. The melting range
tends to be broad and too low. No attempt to purify the products but recrystallization has been done. Analysis by tlc shows a major product and some
minor spots.
No further work is planned at this time to optimize the reaction or purify the product. However, I do believe that this preparation can be carried out
by the home chemist to obtain an interesting product. There are a number of literature reports utilizing this product as an intermediate in organic
synthesis.
Experimental
General: Glutamic acid was USP grade obtained from a supplement company. Its melting point was consistent with that reported in the literature. Ethyl
acetate, sodium nitrite, sodium bisulfate and ammonium sulfate were obtained from reliable suppliers. The sodium bisulfate used was given a label
assay of 93.2%. The amount of sodium bisulfate shown below is “pure” compound after assay correction.
Technical note: It is important to introduce the sodium nitrite solution well below the surface of the reaction solution. In these experiments, this
was accomplished by attaching a disposable glass pipet to the outlet of a separatory funnel. The end of the pipet had a shallow bend about 1.5 cm from
its end and was immersed to nearly the bottom of the reaction solution. If the sodium nitrite solution is added by dropping directly into the
reaction, significant amounts of nitrogen oxides are produced.
General procedure for each experiment: Glutamic acid (7.35 g, 50 mmole) was added to a solution of sodium bisulfate (6.72 g, 52 mmole) in water
(100ml). The magnetically stirred mixture, contained in a 250 ml beaker held by a suitable clamp, was immersed in an oil bath and heated to 85 C
giving a clear solution. Sodium nitrite (3.53 g, 51 mmole) was dissolved in water (25 ml) and charged to a 60 ml separatory funnel fitted as described
above. The nitrite solution was added at such a rate that no nitrogen oxides could be detected (sight and odor). Nitrogen is continuously evolved as
long as glutamate is present. The addition required about 35 minutes and then the reaction was stirred until no further nitrogen was visibly evolved,
about 20 minutes. At this point, the reaction solution was tested with starch-iodide paper. If the test was positive, urea was added a spatula tip at
a time until the starch iodide test was negative. The reaction was then cooled to room temperature for work up.
Experiment 1: The reaction solution was concentrated to about 30 ml at aspirator pressure (ca 20 mm, circulating system) using normal distillation
equipment at a maximum bath temperature of 90C. This distillation took about 2 hours. Upon cooling the mixture partially solidified. The mixture was
washed onto a sinter funnel with ethyl acetate (ca 50 ml). The solids were further washed with ethyl acetate (2 x 25 ml) and the combined ethyl
acetate washings were dried overnight using sodium sulfate. The mixture was filtered and concentrated by distillation. The yellow oil pot residue was
then further dried under aspirator vacuum during which time it crystallized to a waxy solid (4.75 g, 75%). The melting range was found to be 59C –
65C.
Experiment 2: After the reaction was complete, the reaction solution was heated on a hot plate with good stirring until the volume was reduced to
about 50 ml. This concentrate on cooling to ambient was viscous and slowly deposited a crystalline solid which was identified as sodium sulfate
decahydrate. This mixture was transferred to a separatory funnel and extracted with ethyl acetate (50 ml) followed by two additional extractions (2 x
25 ml). After drying and concentration a pale yellow oil was obtained which crystallized after pumping at aspirator vacuum (2.78 g, 43%). Further
extraction of the aqueous solution with ethyl acetate (3x20 ml) afforded additional crystalline product (0.74 g, 11%) for a combined yield of 54%.
Experiment 3: The cooled reaction solution was treated with ammonium sulfate (35 g) and extracted with ethyl acetate (4x30 ml). The extracts were
dried and concentrated to afford crystalline product (2.51 g, 38%). The aqueous solution was extracted again (4x30 ml) which after drying and
concentration gave a crystalline product (1.52 g, 24%). A third extraction (4x30 ml) provided a colorless crystalline solid (0.69 g, 11%). The solids
were combined in ethyl acetate and reisolated (4.72 g, 73%) with a melting range of 51C-56C.
Reported melting point of 5-oxo-2-tetrahydrofurancarboxylic acid: 50C [1], 71C-73C [2], 71C [3], 65C-68C [7].
All three experimental products appeared to be largely a single component by tlc:
Silica gel, 95% ethanol (78%): 10% ammonium hydroxide (22%). Rf 0.48 – 0.57 (elongated spot by iodine visualization).There was also faster running
material appearing as a “smear” by iodine.
References
1. Austin, AT and Howard, J., J Chem. Soc. 3593 (1961)
2. Ravid, U., et al., Tetrahedron, v34, 1449 (1978)
3. Herdeis, C., Synthesis, 232 (1986)
4. Markgraf, JH and Davis, HA, J. Chem. Educ., v67, 173 (1990). This reference provides a “student” level preparation.
5. US Patent 2,461,701
6. Japanese patent JP10237059: Preparation of 5-oxo-2-tetrahydrofurancarboxylic acid and its purification by solvent extraction
• Yamagata, Kazuyuki; Ueda, Hiroshi
• From Jpn. Kokai Tokkyo Koho (1998), JP 10237059 A Sep 08, 1998. | Language: Japanese
High-purity 5-oxo-2-tetrahydrofurancarboxylic acid (I) is prepd. by treatment of glutamic acid-H2O mixt. with HNO2, then extn. of the aq. reaction
mixt. with H2O-immiscible org. solvents in the presence of MgSO4, Na2SO4, (NH4)2SO4, CaCl2, and/or MgCl2. An aq. soln. contg. (S)-I and L-glutamic
acid was treated with MgSO4.7H2O and HCl, then extd. with AcOEt to give (S)-I with 98% purity and 92% recovery.
7. Cooke, RC, J. Agric. Food Chem., v57, 2462 (2009)
AvB
[Edited on 4-3-2017 by AvBaeyer]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Nice writeup. One comment: in your mechanism drawing, you should show a positive charge on the diazonium group.
For TLC, you might also try adding acetic or formic acid (instead of ammonia) to your eluent, to try to keep everything protonated instead of
deprotonated.
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Beautiful writeup, neat experiment, I'll definitely add this to my ever growing to-do list.
Why did you abandon your intended use for it if you don't mind me asking?
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
I have added references to literature mps for the target compound which I forgot in the initial post.
Metacelsus: The + got lost somewhere along the line. As for tlc, this was the simplest system that was referenced in "Thin layer Chromatography" by E.
Stahl (the chromatography bible). I am aware of using acid based systems but I am running low on tlc plates and did not want to do too much
experimenting after I decided to not use the compound.
JnPS: I abandoned the use of the title compound because I was able to obtain a far more advanced intermediate at a relatively cheap price. The
4-penten-1-ol is itself an intermediate in a much longer synthesis so I need a fair amount. I will report on this in due course. Of course, I can
purchase the 4-penten-1-ol but at a painful price. Besides, it's more fun to make it.
AvB
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Does this synthesis extend to glutamines? If so you could achieve a surprisingly easy 2-step conversion of the popular supplement theanine to
N-ethylpyrrolidine with decarboxylation.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
won't the nitrous acid react with the amide N and form N-nitroso products ?
You have yourself given a reference for a similar reaction 
http://www.sciencemadness.org/talk/viewthread.php?tid=69346#...
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
The use of glutamine results in the lactone product, see ref 1 in my post. Cyclization does not proceed via the amide nitrogen to the pyrrolidone. Use
of theanine would also provide the lactone, not the lactam. See reference 1 for a thorough explanation.
AvB
|
|
|
|